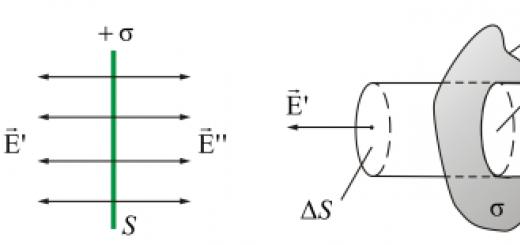
It is an example of a well-defined enantiotropic polymorphism. It is known in three crystalline modifications included in the sulfur group: α-sulfur, β-sulfur (sulfurite), γ-sulfur (rosickite). The most stable modification under normal conditions is rhombic (α-sulfur), which includes natural sulfur crystals. The second, monoclinic modification (β-sulfur) is the most stable at high temperatures. Monoclinic, when cooled to a temperature of 95.5 ° C, becomes rhombic. In turn, rhombic, when heated to this temperature, becomes monoclinic and melts at a temperature of 119 ° C. Distinguish between crystalline and amorphous sulfur. Crystalline sulfur dissolves in organic compounds (turpentine, carbon disulfide and kerosene), while amorphous sulfur does not dissolve in carbon disulfide. Amorphous sulfur impurities reduce the melting point of crystalline sulfur and make it difficult to purify.
Chemical composition
. Sulfur is often found chemically pure, sometimes contains up to 5.2% selenium (selenium sulfur), as well as. Very often, sulfur is contaminated with mechanical impurities of clay and bituminous substances.
The structural cell contains 128S. space group D242h- FDD; and 0 = 10.48, b 0 =12,92 with 0 = 24,55; a 0: b 0: c 0 = 0.813:1.1:1.903. The structure of rhombic sulfur is based on a complex molecular lattice. Elementary the cell consists of 16 electrically neutral molecules, united in a chain of closed, zigzag "wrinkled" rings of 8 sulfur atoms
s - s - 2.12A, s 8 - s 8 \u003d 3.30 A
Aggregates and habitus . Sulfur occurs in the form of pilaf and earthy accumulations, as well as druses of crystals, sometimes in the form of sinter forms and blooms. Often there are well-formed crystals of bipyramidal (elongated bipyramidal and cut bipyramidal) and tetrahedral habitus, the size of which reaches several centimeters. The main forms on rhombic sulfur crystals are bipyramids (111), (113), prisms (011), (101) and pinacoid (001).
Less common, but characteristic of some deposits, are pinacoidal crystals (table and lamellar appearance). Occasionally, there are twins of sulfur intergrowth along (111), sometimes along (011) and (100). Quite often, sulfur crystals form parallel growths.
Physical properties . Sulfur is characterized by different shades of yellow, rarely brown to black. The color of the line is yellowish. The brilliance on the edges is diamond, on the break - bold. Shines through in crystals. Cleavage imperfect in (001), (110), and (111). Hardness-1-2. Fragile. Density - 2.05-2.08. Sulfur is a good thermal insulator. It has semiconductor properties. When rubbed, it is charged with negative electricity.
Optically positive; 2V = 69°; ng - 2.240 - 2.245, nm - 2.038. np = 1.951 - 1.958, ng - np = 0.287.
Diagnostic features
. Crystalline forms, color, low hardness and density, oily sheen at the break of crystals, low temperature melting - characteristics sulfur. Main lines on radiographs: 3.85; 3.21 and 3.10. In HCl and H 2 S0 4 it is insoluble. NH0 3 and aqua regia oxidize sulfur, turning it into H 2 S0 4 . Sulfur dissolves easily in carbon disulfide, turpentine and kerosene. P. p. t. easily melts and ignites with a blue flame with the release of S0 2.
Education and deposits. Sulfur is widespread in nature, its deposits appear: 1) during volcanic eruptions; 2) during the surface decomposition of sulfosalts and sulfur compounds of metals, 3) during the deoxidation of sulfate compounds(mainly gypsum), 4) upon destruction organic compounds(mainly sulfur-rich asphalts and oil), 5) during the destruction of organic organisms and 6) during the decomposition of hydrogen sulfide (as well as S0 2) on the earth's surface. Regardless of these processes, sulfur is formed due to hydrogen sulfide and sometimes S0 2 and S0 3, which are intermediate products during the decomposition of other sulfur formations.
Industrial deposits sulfur is represented by three types: 1) volcanic deposits, 2) deposits associated with the oxidation of sulfides, and 3) sedimentary deposits. Volcanic sulfur deposits arise by crystallization of sublimates. Sulfur in the form of well-formed crystals lines the outlets of fumaroles and small cracks and voids. Volcanic sulfur deposits are known in Italy, Japan, Chile and other volcanic regions. In the Soviet Union, they are found in Kamchatka and the Caucasus. Sulfur deposits associated with the oxidation of sulfides are typical for the zone of oxidation of sulfide deposits. Their formation is due to the incomplete oxidation of sulfides and the first stage of oxidation occurs according to such a possible reaction:
RS + Fe 2 (S0 4 ) 3 = 2FeS0 4 + RS0 4 + S.
Sulfur deposits, which arose during the formation of sedimentary rocks, are of the greatest importance in terms of reserves. In these deposits, the initial substance for the formation of sulfur is. The oxidation of hydrogen sulfide occurs as follows:
2HS + 0 2 \u003d 2H 2 0 + 2S.
As for the origin of hydrogen sulfide itself and the ways of its transition to sulfur, most scientists consider these processes from a biochemical point of view, linking them with the vital activity of organisms. At the end of the 19th century, a number of microbes were discovered, which are characterized by the ability to process (restore) sulfate salts in. At the same time, it has been established that it is formed during the decay of protein compounds and as a result of the vital activity of some types of radiant fungus
Actynomics. Among the microbes, the genus Microspira stands out, which inhabits the bottom of stagnant reservoirs and sea basins contaminated with hydrogen sulfide. These organisms are also found in underground waters and oil at depths up to 1000-1500 m. due to oxygen, which they receive from sulfates (for example, gypsum). In this case, the whole process of hydrogen sulfide formation is as follows:
Ca²⁺+ SO²⁻ 4 + 2C + 2H 2 0 \u003d H 2 S + Ca (HC0 3) 2
The transition of hydrogen sulfide to sulfur can occur either by the reaction 2H 2 S + O 2 \u003d 2H 2 0 + 2S, or biochemically under the influence of other bacteria, the most important of which are Biggiatoa mirabith Thiospirillitis. These bacteria, absorbing hydrogen sulfide, process it into sulfur, which is deposited inside their cells in the form of yellow shiny balls. Bacteria live in lakes, ponds and shallow parts of the sea and, falling to the bottom along with other sediments, give rise to sulfur deposits.
Place of Birth, in which sulfur occurs simultaneously with the rocks that contain it, are called syngenetic. They are known in Sicily, in the Soviet Union (in Turkmenistan, the Volga region, Dagestan, Transnistria and other places). A feature of syngenetic sulfur deposits is its close connection with a certain stratigraphic horizon. When sulfur is formed from hydrogen sulfide, which circulates through the cracks of rocks, epigenetic deposits arise. These include the Texas and Louisiana fields in the US; in Russia - Shor-Su in Fergana, as well as deposits in the region of Makhachkala, Kazbek and Grozny. Many of these deposits are characterized by recrystallization phenomena, which result in the formation of large-crystalline accumulations of sulfur. For example, in the Rozdol deposit, primary sulfur is represented by a cryptocrystalline difference, and secondary (recrystallized) sulfur is a coarse-grained difference with individual crystals up to 5 cm.
In Russia, sulfur deposits are developed in Transnistria, where sulfur occurs in the gypsum-limestone strata of the Upper Tortonian in the form of cryptocrystalline accumulations in pelitomorphic limestone (Rozdolskoye and Yazovskoye deposits), as well as in the form of large crystals in voids in close association with celestite and coarse-grained calcite (Rozdol deposit). AT Central Asia(Gaurdak and Shor-Su), sulfur is observed in cracks and voids of various sedimentary rocks in association with bitumen, gypsum, celestite, calcite, and aragonite. In the Karakum, in the form of hills covered with siliceous rocks in association with gypsum, alum, quartz, chalcedony, etc. Sedimentary sulfur deposits are known in the Volga region. Large sulfur deposits abroad are known in Sicily, as well as in the US in the states of Texas and Louisiana, where they are associated with salt domes.
Chalcogens are a group of elements to which sulfur belongs. Her chemical sign- S - the first letter of the Latin name Sulfur. Compound a simple substance are written using this symbol without an index. Consider the main points regarding the structure, properties, production and use of this element. The characterization of sulfur will be presented in as much detail as possible.
Common features and differences of chalcogens
Sulfur belongs to the oxygen subgroup. This is the 16th group in the modern long period image form. periodic system(PS). An obsolete version of the number and index is VIA. Titles chemical elements groups, chemical signs:
- oxygen (O);
- sulfur (S);
- selenium (Se);
- tellurium (Te);
- polonium (Po).
External electron shell of the above elements is arranged in the same way. In total it contains 6 who can participate in education chemical bond with other atoms. Hydrogen compounds correspond to the composition H 2 R, for example, H 2 S - hydrogen sulfide. The names of the chemical elements that form two types of compounds with oxygen: sulfur, selenium and tellurium. The general formulas of the oxides of these elements are RO 2, RO 3.
Chalcogens correspond to simple substances that differ significantly in physical properties. Most common in earth's crust of all chalcogens, oxygen and sulfur. The first element forms two gases, the second - solids. Polonium, a radioactive element, is rarely found in the earth's crust. In the group from oxygen to polonium non-metallic properties decrease and increase metal. For example, sulfur is a typical non-metal, while tellurium has a metallic luster and electrical conductivity.
Element No. 16 of the D.I. Mendeleev
The relative atomic mass of sulfur is 32.064. Of the natural isotopes, 32 S is the most common (more than 95% by weight). Nuclides are found in smaller quantities atomic mass 33, 34 and 36. Characteristics of sulfur by position in PS and atomic structure:

- serial number - 16;
- the charge of the nucleus of an atom is +16;
- atomic radius - 0.104 nm;
- ionization energy -10.36 eV;
- relative electronegativity - 2.6;
- oxidation state in compounds - +6, +4, +2, -2;
- valency - II (-), II (+), IV (+), VI (+).
Sulfur is in the third period; The electrons in an atom are arranged in three energy levels: on the first - 2, on the second - 8, on the third - 6. All external electrons are valence. When interacting with more electronegative elements, sulfur gives up 4 or 6 electrons, acquiring typical degrees oxidation +6, +4. In reactions with hydrogen and metals, the atom attracts the missing 2 electrons until the octet is filled and a steady state is reached. in this case it drops to -2.
Physical properties of rhombic and monoclinic allotropic forms

Under normal conditions, sulfur atoms are connected to each other at an angle into stable chains. They can be closed in rings, which allows us to speak about the existence of cyclic sulfur molecules. Their composition reflect the formulas S 6 and S 8 .
The characterization of sulfur should be supplemented by a description of the differences between allotropic modifications with different physical properties.
Rhombic or α-sulfur is the most stable crystalline form. These are bright yellow crystals composed of S 8 molecules. The density of rhombic sulfur is 2.07 g/cm3. Light yellow monoclinic crystals are formed by β-sulfur with a density of 1.96 g/cm3. The boiling point reaches 444.5°C.
Obtaining amorphous sulfur
What color is sulfur in the plastic state? It is a dark brown mass, completely different from yellow powder or crystals. To obtain it, you need to melt rhombic or monoclinic sulfur. At temperatures above 110°C, a liquid is formed, with further heating it darkens, at 200°C it becomes thick and viscous. If you quickly pour molten sulfur into cold water, then it will solidify with the formation of zigzag chains, the composition of which is reflected by the formula S n.

Solubility of sulfur
Some modifications in carbon disulfide, benzene, toluene and liquid ammonia. If organic solutions are cooled slowly, needle-like crystals of monoclinic sulfur are formed. When liquids evaporate, transparent lemon-yellow crystals of rhombic sulfur are released. They are brittle and can be easily ground into powder. Sulfur does not dissolve in water. The crystals sink to the bottom of the vessel, and the powder can float on the surface (not wetted).

Chemical properties
The reactions show the typical non-metallic properties of element No. 16:
- sulfur oxidizes metals and hydrogen, is reduced to the S 2- ion;
- when burned in air and oxygen, di- and sulfur trioxide are formed, which are acid anhydrides;
- in a reaction with another more electronegative element - fluorine - sulfur also loses its electrons (is oxidized).
Free sulfur in nature
In terms of prevalence in the earth's crust, sulfur is in 15th place among the chemical elements. The average content of S atoms in is 0.05% of the mass of the earth's crust.
What color is sulfur in nature (native)? It is a light yellow powder with a characteristic odor or yellow crystals with a glassy luster. Deposits in the form of placers, crystalline layers of sulfur are found in areas of ancient and modern volcanism: in Italy, Poland, Central Asia, Japan, Mexico, and the USA. Often, when mining, beautiful druze and giant single crystals are found.
Hydrogen sulfide and oxides in nature
In areas of volcanism, gaseous sulfur compounds come to the surface. The Black Sea at a depth of over 200 m is lifeless due to the release of hydrogen sulfide H 2 S. The formula of sulfur oxide is bivalent - SO 2, trivalent - SO 3. The listed gaseous compounds are present in some oil, gas, natural waters. Sulfur is part of coal. It is necessary for the construction of many organic compounds. When egg whites rot, hydrogen sulfide is released, which is why it is often said that this gas has the smell of rotten eggs. Sulfur is a biogenic element, it is necessary for the growth and development of humans, animals and plants.

Importance of natural sulfides and sulfates
The characterization of sulfur will be incomplete, if not to say that the element occurs not only in the form of a simple substance and oxides. The most common natural compounds are salts of hydrosulfide and sulfuric acids. Sulfides of copper, iron, zinc, mercury, lead are found in the minerals sphalerite, cinnabar and galena. Sulfates include sodium, calcium, barium and magnesium salts, which form minerals and rocks in nature (mirabilite, gypsum, selenite, barite, kieserite, epsomite). All these compounds are used in various sectors of the economy, used as raw materials for industrial processing, fertilizers, building materials. The medical value of some crystalline hydrates is great.
Receipt
A yellow substance in a free state occurs in nature at different depths. If necessary, sulfur is smelted from rocks, without raising them to the surface, but by forcing superheated rocks to a depth. Another method is associated with sublimation from crushed rocks in special furnaces. Other methods involve dissolution with carbon disulfide or flotation.
The needs of industry for sulfur are great, therefore, its compounds are used to obtain elemental matter. In hydrogen sulfide and sulfides, sulfur is in reduced form. The oxidation state of the element is -2. Sulfur is oxidized, increasing this value to 0. For example, according to the Leblanc method, sodium sulfate is reduced with coal to sulfide. Then calcium sulfide is obtained from it, processed carbon dioxide and water vapor. The resulting hydrogen sulfide is oxidized with atmospheric oxygen in the presence of a catalyst: 2H 2 S + O 2 = 2H 2 O + 2S. The determination of sulfur obtained by various methods sometimes gives low purity values. Refining or purification is carried out by distillation, rectification, treatment with mixtures of acids.

The use of sulfur in modern industry
Sulfur granulated is used for various production needs:
- Obtaining sulfuric acid in the chemical industry.
- Production of sulfites and sulfates.
- Production of preparations for plant nutrition, control of diseases and pests of agricultural crops.
- Sulfur-containing ores are processed at mining and chemical plants to obtain non-ferrous metals. Accompanying production is sulfuric acid.
- Introduction to the composition of some grades of steels to impart special properties.
- Thanks get rubber.
- Manufacture of matches, pyrotechnics, explosives.
- Use for the preparation of paints, pigments, artificial fibers.
- Bleaching fabrics.

Toxicity of sulfur and its compounds
Dust-like particles with an unpleasant odor irritate the mucous membranes of the nasal cavity and respiratory tract, eyes, and skin. But the toxicity of elemental sulfur is not considered particularly high. Inhalation of hydrogen sulfide and dioxide can cause severe poisoning.
If, during the roasting of sulfur-containing ores at metallurgical plants, exhaust gases are not captured, then they enter the atmosphere. Combining with drops and water vapor, sulfur and nitrogen oxides give rise to the so-called acid rain.
Sulfur and its compounds in agriculture
Plants absorb sulfate ions along with the soil solution. A decrease in sulfur content leads to a slowdown in the metabolism of amino acids and proteins in green cells. Therefore, sulfates are used for fertilizing crops.

To disinfect poultry houses, basements, vegetable stores, a simple substance is burned or the premises are treated with modern sulfur-containing preparations. Sulfur oxide has antimicrobial properties, which has long been used in the production of wines, in the storage of vegetables and fruits. Sulfur preparations are used as pesticides to control diseases and pests of crops (powdery mildew and spider mites).
Application in medicine

Great importance the great healers of antiquity Avicenna and Paracelsus gave the study of the healing properties of yellow powder. Later it was found that a person who does not receive enough sulfur with food becomes weaker, experiences health problems (these include itching and flaking of the skin, weakening of hair and nails). The fact is that without sulfur, the synthesis of amino acids, keratin, and biochemical processes in the body is disrupted.
Medical sulfur is included in ointments for the treatment of skin diseases: acne, eczema, psoriasis, allergies, seborrhea. Sulfur baths can relieve the pain of rheumatism and gout. For better absorption by the body, water-soluble sulfur-containing preparations have been created. This is not a yellow powder, but a fine crystalline substance white color. When used externally, this compound is incorporated into a skin care cosmetic.
Gypsum has long been used in the immobilization of injured parts of the human body. prescribed as a laxative. Magnesia lowers blood pressure, which is used in the treatment of hypertension.
Sulfur in history
Even in ancient times, a non-metallic yellow substance attracted the attention of a person. But it wasn't until 1789 that the great chemist Lavoisier established that powders and crystals found in nature were composed of sulfur atoms. It was believed that the unpleasant smell that occurs when it is burned, repels all evil spirits. The formula for sulfur oxide, which is obtained during combustion, is SO 2 (dioxide). It is a toxic gas and is hazardous to health if inhaled. Several cases of mass extinction of people by entire villages on the coasts, in the lowlands, scientists explain the release of hydrogen sulfide or sulfur dioxide from the earth or water.
The invention of black powder increased military interest in yellow crystals. Many battles were won thanks to the ability of craftsmen to combine sulfur with other substances in the manufacturing process. The most important compound - sulfuric acid - also learned to use a very long time ago. In the Middle Ages, this substance was called vitriol oil, and salts were called vitriol. Copper sulphate CuSO 4 and ferrous sulphate FeSO 4 still have not lost their importance in industry and agriculture.
sulfur crystal
Native sulfur(Sulfur) is a beautiful light yellow, lemon yellow, rich yellow crystals that will adorn any collection of minerals with their sunny color. Aggregates of a brownish color are encountered; this coloration is imparted by an admixture of organic matter. Sulfur crystals can be a fraction of millimeters in size, but they can also reach quite large sizes - up to tens of centimeters.

lemon yellow sulfur crystals
physical properties. Sulfur crystals have a monoclinic or rhombic syngony, the appearance of crystals is a combination of a cube and a rhombus. The crystals have a glassy shine. Hardness of this mineral is small - 1-2 units on the ten-point Mohs scale. Density sulfur crystals is 2.05 - 2.08 grams per cubic centimeter. The crystals are brittle and break easily on impact.
Sulfur crystals have low electrical conductivity and can be used as an electrical insulator. This mineral conducts heat poorly and is an excellent thermal insulator.
Native sulfur may contain, selenium, astatine and tellurium as an isomorphic impurity. When rubbed, this mineral becomes charged with static electricity and can attract light objects, such as small pieces of paper, to itself.
Sulfur can be melted by heating it to a temperature of 115.2 degrees Celsius; when exposed to high temperatures, this mineral actively oxidizes and burns. At the same time, it highlights sulfuric anhydride SO3- gas with a suffocating odor.
Alchemists used native sulfur in their experiments, and this occupation among the population of European countries of that time was equal to witchcraft. In the Middle Ages and the Renaissance, the Inquisition organized a real hunt for witches and magicians. Therefore, the smell of burning sulfur has become associated with evil spirits and the devil.

Islets of sulfur, Dallol volcano, Ethiopia
Sulfur formed during the weathering of sulfide deposits of metals.
In the vicinity of some damped (or cooled down) volcanoes, solutions saturated with metal sulfide ions come to the earth's surface. When they are deposited, they also form native sulfur (volcanic).
This mineral can also be formed during the decomposition of some salts. So, native sulfur is formed during the oxidation and decomposition of the mineral gypsum(CaSO4 2H2O) . There are such deposits on the southern and northern coasts of Italy.
Sulfur deposits are actively developed both in Russia (the Volga region) and abroad in the United States (in Texas and Louisiana). It is used to produce sulfuric acid.
There are deposits of volcanic sulfur in Japan and Ethiopia. But in these countries, this mineral is not being mined.
Deposits of volcanic sulfur occupy large areas, while yellow, orange, red layers and streams form the most beautiful fantastic landscapes.

A volcanic eruption on Io, the satellite of Saturn (photo by the Voyager spacecraft). The surface of the planet is covered with a layer of sulfur.
Sulfur is a common mineral, both on Earth and on other planets. For example, Saturn's moon And about is a small planet (comparable in volume to the Moon), with a molten core. Volcanoes often erupt on Io; when they cool, a lot of elemental (native) sulfur is released. This mineral makes the surface of the planet look like an egg yolk.
Rocks that make up the crust Venus- mostly gray basalts. But on this planet there are many areas in which volcanoes are active. According to the scan of this planet spacecraft, the surface in these areas is also covered with a layer of native sulfur.

Extraction of native sulfur. USA, Texas.
The crystals of this bright yellow mineral are very beautiful, but the collector should be aware that they are fragile and afraid of exposure to high temperatures.
Sulfur is known in nature in several polymorphic crystalline modifications, in colloidal secretions, in liquid and gaseous states. AT natural conditions stable modification is rhombic sulfur (α-sulfur). At atmospheric pressure at temperatures above 95.6 ° α-sulfur passes into monoclinic β-sulfur, upon cooling it again becomes rhombic. γ-sulfur, which also crystallizes in the monoclinic system, is unstable at atmospheric pressure and transforms into α-sulfur. The structure of γ-sulfur has not been studied; it is conditionally assigned to this structural group.
The article considers several polymorphic modifications of sulfur: α-sulfur, β-sulfur, γ-sulfur
α modification
The English name for the mineral α-sulfur is α-Sulphur
origin of name
The name α-sulfur was introduced by Dana (1892).
Synonyms:
Rhombic sulfur. Usually just called gray. Dayton-sulfur (Suzuki, 1915) - pseudomorphosis of α-sulfur after β-sulfur.
Formula
Chemical composition
Often, native sulfur is practically pure. Sulfur of volcanic origin often contains small amounts of As, Se, Te and traces of Ti. Sulfur from many deposits is contaminated with bitumen, clay, various sulfates and carbonates. It contains inclusions of gases and a liquid containing a mother liquor with NaCl, CaCl, Na2SO4, etc. It sometimes contains up to 5.18% Se (selenium sulfur)
Varieties
1. Volcanite- (selenium sulfur) orange-red, red-brown color.
 Crystallographic characteristic
Crystallographic characteristic
Syngony. Rhombic.
Class. Dipyramidal. Some authors believed that sulfur crystallizes into a rhombo-tetrahedral class, since sometimes it has the form of sphenoids, but this form, according to Royer, is explained by the influence of an asymmetric medium (active hydrocarbons) on crystal growth.
Crystal structure of sulfur
The structure of sulfur is molecular: 8 atoms in the lattice are included in one molecule. The sulfur molecule forms eight-dimensional rings in which the atoms alternate at two levels (along the axis of the ring). 4 S atoms of one level form a square rotated by 45° relative to another square. The planes of the squares are parallel to the c-axis. The centers of the rings are located in a rhombic cell according to the "diamond" law: at the vertices and centers of the faces of the face-centered cell and at the centers of four of the eight octants into which the unit cell is divided. In the structure of sulfur, the Hume-Rothery principle is maintained, requiring coordination 2 (= 8 - 6) for the elements of the Mendeleev group V1b. In the structure of tellurium - selenium, as well as in monoclinic sulfur, this is achieved by a helical arrangement of atoms, in the structure of rhombic sulfur (as well as synthetic β-selenium and β-tellurium) - by their ring arrangement. The S - S distance in the ring is 2.10 A, which is exactly the same as the S - S distance in the S 2 radical of pyrite (and covelline) and a little more distance S-S between S atoms from different rings (3.3 A).
Form of being in nature
Crystal Shape
The shape of the crystals is different - dipyramidal, less often thick tabular along with (001), disphenoidal, etc. On the faces (111), figures of natural etching are observed, which are absent on the faces (113).
Doubles
Twins at (101), (011), (110) or (111) are rare; twins at (211) are also noted.
Aggregates. Solid masses, spherical and kidney-shaped secretions, stalactites and stalagmites, powdery deposits and crystals.
Physical properties
Optical
- The color is sulfur-yellow, straw-and honey-yellow, yellow-brown, reddish, greenish, gray from impurities; sometimes from bitumen impurities the color is brown or almost black.
- The line is colorless.
- Glitter diamond
- The tide is resinous to greasy.
- Transparency. Transparent to translucent.
Mechanical
- Hardness 1-2. Fragile.
- Density 2.05-2.08.
- Cleavage by (001), (110), (111) imperfect. Separateness according to (111).
- The fracture is conchoidal to uneven.
Chemical properties
Soluble in carbon disulfide, turpentine, kerosene.
Other properties
The electrical conductivity at ordinary temperature is almost zero. With friction sulfur electrified negatively. In ultraviolet rays, a plate 2 mm thick is opaque. At atmospheric pressure, the melting temperature 112.8°; boiling point + 444.5 °. Heat of fusion at 115° 300 cal/g-atom. Heat of vaporization at 316° 11600 cal/g-atom. At atmospheric pressure at 95.6°, α-sulfur transforms into β-sulfur with an increase in volume.

artificial receiving
Obtained by sublimation or crystallization from solution.
Diagnostic features
Easily recognized by yellow, brittleness, gloss and flammability.
Associated minerals. Gypsum, anhydrite, opal, jarosite, asphalt, petroleum, ozokerite, hydrocarbon gas, hydrogen sulfide, celestine, halite, calcite, aragonite, barite, pyrite
Origin and location in nature
Native sulfur is found only in the uppermost part of the earth's crust. Formed in a variety of processes.
Animal and plant organisms play an important role in the formation of sulfur deposits, on the one hand, as S accumulators, and on the other, as contributing to the breakdown of H 2 S and other sulfur compounds. The formation of sulfur in waters, silts, soils, swamps and in oils is associated with the activity of bacteria; in the latter, it is partly contained in the form of colloidal particles. Sulfur can be released from waters containing H 2 S under the influence of atmospheric oxygen. In coastal areas, sulfur precipitates in places when fresh water is mixed with salt water (from H 2 S sea water, under the action of oxygen dissolved in fresh waters). From some natural waters, sulfur is released in the form of white turbidity (the Molochnaya river in the Kuibyshev region, etc.). From the waters of sulfur sources and from swamp waters containing H 2 S and S, sulfur precipitates in the northern regions of Russia in the winter during the process of freezing. One way or another, the main source of sulfur formation in many deposits is H 2 S, whatever its origin.
Significant accumulations of sulfur are observed in volcanic areas, in the zone of oxidation of some deposits and among sedimentary strata; deposits of the last group serve as the main sources of native sulfur mined for practical purposes. In volcanic areas, sulfur is released both during volcanic eruptions and from fumaroles, solfataras, hot springs and gas jets. Sometimes a molten mass of sulfur pours out of the crater of a volcano in the form of a stream (in Japan), and at first β- or γ-sulfur is formed, which later turns into α-sulfur with a characteristic granular structure. During volcanic eruptions, sulfur mainly arises from the action of released H 2 S on sulfur dioxide or from the oxidation of hydrogen sulfide with atmospheric oxygen; it can also sublimate with water vapor. Vapors S can be captured by gases of fumaroles, jets of carbon dioxide. First observed stage volcanic eruptions the blue flame represents clouds of burning sulfur (Vulcano, in the Aeolian Islands, Italy). The hydrogen sulfide stage of fumaroles and solfataras, accompanied by the formation of native sulfur, follows after the stage of isolation of fluorine and chloride compounds and precedes the stage of carbon dioxide emissions. Sulfur is released from solfataras in the form of loose tuff-like products, which are easily transported by wind and precipitation, forming secondary deposits (Cove Creek, Utah, USA).  Sulfur. Crystals in plaster
Sulfur. Crystals in plaster
Mineral change
In the earth's crust native sulfur easily oxidized with the formation of sulfuric acid and various sulfates; under the influence of bacteria can also produce hydrogen sulfide.
Place of Birth
Sulfur deposits of volcanic origin are usually small; they are found in Kamchatka (fumaroles), on Mount Alagez in Armenia, in Italy (solfataras of Slit Pozzuoli), in Iceland, Mexico, Japan, the USA, Java, the Aeolian Islands, etc.
The release of sulfur in hot springs is accompanied by the deposition of opal, CaCO 3 , sulfates, etc. In places, sulfur replaces limestone near hot springs, sometimes it is released in the form of the finest turbidity. Hot springs depositing sulfur are observed in volcanic areas and in areas of young tectonic faults, for example, in Russia - in the Caucasus, Central Asia, Far East, on the Kuril Islands; in the USA - in Yellowstone national park, in California; in Italy, Spain, Japan, etc.
Often native sulfur is formed in the process of supergene changes during the decomposition of sulfide minerals (pyrite, marcasite, melnikovite, galena, antimonite, etc.). Quite large accumulations have been found in the oxidation zone of pyrite deposits, for example, in the Stalinskoye deposit in the Sverdlovsk region. and in the Blyavinsky deposit of the Orenburg region; in the latter, sulfur has the appearance of a dense but brittle mass of layered texture, of various colors. In the Maykain deposit in the Pavlodar region (Kazakhstan), large accumulations of native sulfur were observed between the zone of jarosites and the zone of pyrite ores.
In small quantities, native sulfur is found in the oxidation zone of very many deposits. Sulfur is known to form in connection with coal fires during spontaneous combustion of pyrite or marcasite (powdered sulfur in a number of Ural deposits), during fires in oil shale deposits (for example, in California).
In black sea silt, sulfur is formed when it turns gray in air due to the change in the monosulfuric iron contained in it.
The largest industrial sulfur deposits are found among sedimentary rocks, mainly of Tertiary or Permian age. Their formation is associated with the reduction of sulfur sulfates, mainly gypsum, less often - anhydrite. The question of the origin of sulfur in sedimentary formations is controversial. Gypsum, under the influence of organic compounds, bacteria, free hydrogen, etc., is first reduced, possibly to CaS or Ca(HS) 2 , which, under the action of carbon dioxide and water, transform into calcite with the release of hydrogen sulfide; the latter, when reacting with oxygen, gives sulfur. Accumulations of sulfur in sedimentary strata sometimes have a reservoir character. Often they are confined to salt domes. In these deposits, sulfur is accompanied by asphalt, oil, ozocerite, gaseous hydrocarbons, hydrogen sulfide, celestine, halite, calcite, aragonite, barite, pyrite and other minerals. Pseudomorphoses of sulfur on fibrous gypsum (selenite) are known. In Russia, there are deposits of this type in the region of the Middle Volga (Syukeyevskoe Tatarstan, Alekeeevskoe, Vodinskoe Samara region, etc.), in Turkmenistan (Gaurdak, Karakum), in the Ural-Embensky district of Kazakhstan, where a number of deposits are confined to salt domes, in Dagestan (Avar and Makhachkala groups) and in other areas.
Outside of Russia, large deposits of sulfur confined to sedimentary strata are found in Italy (Sicily, Romagna), the USA (Louisiana and Texas), Spain (near Cadiz) and other countries.
Practical application of sulfur
It is used in a number of industries: in sulfuric acid, paper-cellulose, rubber, colorful, glass, cement, match, leather, etc. Sulfur is of great importance in agriculture as an insectofungicide for pest control on plantations. Grapes, tea, tobacco, cotton , beets, etc. In the form of sulfur dioxide, it is used in refrigeration, is used for bleaching fabrics, for mordant in dyeing and as a disinfectant.

Physical research methods
Differential thermal analysis
Main lines on radiographs:
ancient methods. Melts easily under a blowpipe. Burns with a bluish flame releasing SO 2 . In a closed tube it gives a yellow crystalline sublimation or reddish-brown droplets, light yellow upon cooling.
Crystal optical properties in thin preparations (sections)
Biaxial (+). Density of optical axes (010); Ng - c, Nm = b, Np = a. Refractive index according to Schrauf.
Diagnostic card.
Sulfur crystals from Cozzodisi (Agrigento)
S
Syngony rhombic or monoclinic
Hardness 2
Specific gravity 2-2.1
Cleavage imperfect
Fracture conchoidal
Color yellow, brown
Color in powder white
Resin to oily gloss

Native sulfur - S. Luster is greasy to diamond, the mineral is transparent to translucent. Colors: yellow, becomes gray or brown to black when weathered. The line is light yellow, the fracture is conchoidal, uneven. Very fragile. Cleavage is imperfect. Sulfur is formed as a product of volcanic sublimates; it is also found in biogenic sedimentary deposits.
Crystals (rhombic syngony) pyramidal, barrel-shaped. Frequent splices. The aggregates are continuous, coarse-grained, dense, sometimes earthy (vine-like and kidney-shaped discharges are found), powdery coatings. It is used for the preparation of sulfuric acid, in the rubber industry and for agricultural pest control. Places of distribution: the island of Sicily (Italy), Spain. Poland, CIS, Japan, pcs. Louisiana (USA), Mexico.
Sulfur is an example of polymorphism. In the stable phase (up to 95 o C) rhombic crystal system, in the range up to 119 o C turns into monoclinic. It melts when the temperature rises. In nature, because of this, it is found mainly in a rhombic form. Sulfur forms bipyramidal crystals and granular aggregates. The characteristic color of this mineral is lemon yellow, which can change up to almost black due to bitumen contamination.

Sulfur (yellow). Guam o., Pacific Ocean, USA. 10 cm. Photo: A.A. Evseev.
 Sulfur (English Sulfur, French Sufre, German Schwefel) in its native state, as well as in the form of sulfur compounds, has been known since ancient times. With the smell of burning sulfur, the suffocating effect of sulfur dioxide and the disgusting smell of hydrogen sulfide, people probably met in prehistoric times. Approximately half of the sulfur produced in the world is extracted from natural reserves.
Sulfur (English Sulfur, French Sufre, German Schwefel) in its native state, as well as in the form of sulfur compounds, has been known since ancient times. With the smell of burning sulfur, the suffocating effect of sulfur dioxide and the disgusting smell of hydrogen sulfide, people probably met in prehistoric times. Approximately half of the sulfur produced in the world is extracted from natural reserves.
diagnostic signs.
Fragile, poor conductor of heat; sometimes a touch of the hand is enough to cause the crystal to crack. Charged with electricity when rubbed. It melts at a low temperature, burns in air, releasing the poisonous gas of sulfuric anhydride.
Origin.
Sulfur is a mineral characteristic of sedimentary deposits such as evaporites and direct ("dry") volcanic sublimation, as well as an element of volcanic (thermal) sulfur sources (poisonous water and hot fumes of sulfur and acid). It is believed that it is formed during the decomposition of sulfates, primarily gypsum (with which it most often occurs together), under the influence of bacteria, primarily "thiobacteria". The monoclinic phase is formed during the sublimation of sulfurous acid vapors in a volcanic medium (in solfataras). In the photo - aggregates of sulfur crystals, commonly called "sulfur flowers".
Deposits and application.
Large sulfur deposits have been found in Texas and Louisiana in the roof of salt domes (evaporite deposits) overlain by clay strata. Sulfur in these deposits has practically no impurities, it is extracted by drilling wells into which boiling water is injected. It melts the sulfur, which is then pumped to the surface (Flash method).
Sulfur is also common in Italy along the outcrops of the gypsum sulfur-bearing strata that delineate the Apennines, especially in Romagna, Marche, Calabria and Sicily. Sulfur is interbedded with clayey rocks there, so for its extraction (now stopped) it is required enough hard way. In the sulfur mines of Sicily, the extrusion method was used. Sulfur mined in the mine was melted and poured into large containers.


Other deposits are known in Japan and Indonesia. In Italy, very beautiful crystals of rhombic sulfur are known from Romagna, Marche (Perticara) and Sicily, where they are associated with celestite and aragonite. Monoclinic sulfur has been installed at Campi Flegeri and on the island of Vulcano. Sulfur is used in the chemical industry and for the production of mineral fertilizers.

Sulfur (crystal). Sicily, Italy. 5x2.5 cm. Photo: A.A. Evseev.

Brush of sulfur crystals (60x40 cm) from the island of Sicily (Italy). Photo: V.I. Dvoryadkin.

Sulfur. Druse of dipyramidal crystals on a crystal of colorless gypsum
and inside it. Sicily, Italy. Photo: A.A. Evseev.
Sulfur is a "mineral of beauty" (a joke in the Soviet "zones", 1939-1969 of the 20th century, where the detention of prisoners was, among other things, on sulfur). The sulfur content in the body of an adult is about 0.16% (110 g per 70 kg of body weight). Sulfur is found in all tissues of the body, a lot of it in the muscles, skeleton, liver, nervous tissue, blood - an active metabolism. The superficial layers of the skin are rich in yellow sulfur, where sulfur is part of keratin and melanin. These are sulfides. Sulfur enters the body food products, as part of inorganic and organic compounds. Most of the sulfur enters the body in the composition of amino acids.
The main manifestations of excess sulfur: itching, rash, furunculosis, redness and swelling of the conjunctiva; the appearance of small point defects on the cornea; aches in the eyebrows and eyeballs, a feeling of sand in the eyes; photophobia, lacrimation, general weakness, headaches, dizziness, nausea, catarrh of the upper respiratory tract, bronchitis; hearing loss, indigestion, diarrhea, weight loss; anemia, mental disorders, decreased intelligence. Sulfur - volcanoes and sulfurous springs, sulfur fumes (99.3%). Accumulate - products. Sulfur-containing compounds (sulfites) are one source of excess sulfur intake, and increased sulfite intake is responsible for the increased incidence of asthma.
Signs of sulfur deficiency: constipation, allergies, dullness and hair loss, brittle nails, high blood pressure, joint pain, tachycardia, high blood sugar and high blood triglycerides. Fatty degeneration of the liver, hemorrhages - in the kidneys, disorders of protein and carbohydrate metabolism, overexcitation nervous system, irritability. Sulfur is the mineral that makes garlic the "king of plants".
Sulfur atoms are an integral part of the molecules of essential amino acids (cystine, cysteine, methionine), hormones (insulin, calcitonin), vitamins (biotin, thiamine), glutathione, taurine and other compounds important for the body. In their composition, sulfur is involved in redox reactions, tissue respiration, energy production, transfer of genetic information, and performs many other important functions. Sulfur is a component of the structural protein of collagen. Chondroitin sulfate is present in the skin, cartilage, nails, ligaments and myocardial valves. Sulfur-containing metabolites are hemoglobin, heparin, cytochromes, fibrinogen and sulfolipids.
Sulfur is excreted in the urine in the form of neutral sulfur and inorganic sulfates, a minor part of the sulfur is excreted through the skin and lungs, and is excreted mainly in the urine as SO42–. Endogenous sulfuric acid, formed in the body, takes part in the neutralization of toxic compounds (phenol, indole, etc.), which are produced by the intestinal microflora, and also binds substances foreign to the body, including drugs and their metabolites. In this case, harmless compounds are formed - conjugates, which are then excreted from the body. Sulfur metabolism is controlled by those factors that have a regulatory effect on protein metabolism (hormones of the pituitary, thyroid, adrenal, gonads).
ADR 2.1
flammable gases
Fire risk. Risk of explosion. May be under pressure. Choking risk. May cause burns and/or frostbite. Capacities can explode when heated (super-dangerous - practically do not burn)
ADR 2.2
gas cylinder Non-flammable, non-toxic gases.
Choking risk. May be under pressure. May cause frostbite (similar to a burn - pallor, blisters, black gas gangrene - creaking). Containers can explode when heated (super-dangerous - an explosion from a spark, flame, match, practically does not burn)
Use cover. Avoid low surface areas (holes, lowlands, trenches)
Green rhombus, ADR number, black or white gas cylinder (such as "cylinder", "thermos")
ADR 2.3
Toxic gases. Skull and crossbones
Danger of poisoning. May be under pressure. May cause burns and/or frostbite. Containers can explode when heated (super-dangerous - instant spread of gases around the area)
Use an emergency escape mask vehicle. Use cover. Avoid low surface areas (holes, lowlands, trenches)
White diamond, ADR number, black skull and crossbones
ADR 3
Flammable liquids
Fire risk. Risk of explosion. Containers may explode when heated (super hazardous - easy to burn)
Use cover. Avoid low surface areas (holes, lowlands, trenches)
Red diamond, ADR number, black or white flame
ADR 4.1
Flammable solids, self-reactive substances and solid desensitized explosives
Fire risk. Flammable or combustible substances can be ignited by sparks or flames. May contain self-reactive substances capable of exothermic decomposition in case of heating, contact with other substances (such as: acids, compounds heavy metals or amines), friction or impact.
This may result in the evolution of harmful or flammable gases or vapours, or self-ignition. Capacities can explode when heated (super-dangerous - practically do not burn).
Risk of explosion of desensitized explosives after loss of desensitizer
Seven vertical red stripes on a white background, equal area, ADR number, black flame
ADR 8
Corrosive (caustic) substances
Risk of burns due to skin corrosion. They can react violently with each other (components), with water and other substances. Spilled/scattered material may release corrosive vapours.
They pose a risk to water environment or sewer system
White upper half of the rhombus, black - lower, equal in size, ADR number, test tubes, hands
| Name of especially dangerous cargo during transportation | Number UN | Class ADR |
| Sulfuric anhydride, stabilized SULFUR TRIOXIDE, STABILIZED | 1829 | 8 |
| Serist anhydride SULFUR DIOXIDE | 1079 | 2 |
| Carbon disulfide | 1131 | 3 |
| Gas SULFUR HEXAFLUORIDE | 1080 | 2 |
| SULFURIC ACID | 1832 | 8 |
| SULFURIC ACID FUMING | 1831 | 8 |
| SULFURIC ACID, which contains not more than 51% acid, or ACID BATTERY LIQUID | 2796 | 8 |
| SULFURIC ACID, REGENERATED FROM ACID TARS | 1906 | 8 |
| SULFURIC ACID, which contains more than 51% acid | 1830 | 8 |
| SULFURIC ACID | 1833 | 8 |
| SULFUR | 1350 | 4.1 |
| SULFUR IS MELTED | 2448 | 4.1 |
| Sulfur chloride SULFUR CHLORIDES | 1828 | 8 |
| Sulfur hexafluoride SULFUR HEXAFLUORIDE | 1080 | 2 |
| Sulfur dichloride | 1828 | 8 |
| SULFUR DIOXIDE | 1079 | 2 |
| SULFUR TETRAFLUORIDE | 2418 | 2 |
| SULFUR TRIOXIDE, STABILIZED | 1829 | 8 |
| SULFUR CHLORIDES | 1828 | 8 |
| hydrogen sulfide | 1053 | 2 |
| CARBON SULFUR | 1131 | 3 |
| SAFE MATCHES in boxes, books, cartons | 1944 | 4.1 |
| PARAFFIN MATCHES „VESTA” | 1945 | 4.1 |
| Paraffin matches PARAFFIN MATCHES „VESTA” | 1945 | 4.1 |
| MATCHES | 2254 | 4.1 |
Stone, mineral, minerals, stones, crystal, rock, precious stones, natural stones, rocks, gemstone, rock, wild stone, stones and minerals, name of stones, natural stone, natural stone, stones minerals, semi-precious stone, minerals these are stones catalog, mineralogy, the meaning of stones, what are minerals, properties of stones, the name of stones and minerals, natural stones names and photos, natural stones, minerals stones, natural stones, stones photos and names, minerals names, wild stone photo, rocks and minerals, minerals and stones, chemical composition minerals, what the stone consists of, the most amazing stones and minerals, minerals list, catalog of minerals, stones and their properties, precious minerals, natural stone, minerals types, types of minerals, stone crystal, stones properties, geology stones, basic minerals, minerals and their classification, the most beautiful minerals, minerals definition, the origin of stones, crystal mineral, ordinary stones, minerals classification, stones description, what gems look like in nature, stone what is it, types of natural stone, valuable mineral, mineral science, chemical classification minerals, magnetic properties minerals, world of minerals, mineral rock, what rocks and minerals are, types of stones, stone composition, description of minerals, stones in nature, useful stones, determinant of stones, density of minerals, hardness of rocks, pictures of stones and their names, classification of minerals geology, rocks and minerals, semi-precious stones names and photos, characteristics of minerals, stone structure, minerals in nature.