Electrical conductivity characterizes the ability of a body to conduct an electric current. Conductivity - resistance value. In the formula, it is inversely proportional to electrical resistance, and they are actually used to refer to the same material properties. Conductivity is measured in Siemens: [cm]=.
Types of electrical conductivity:
— Electronic conductivity where the charge carriers are electrons. Such conductivity is typical primarily for metals, but is present to one degree or another in almost all materials. With increasing temperature, the electronic conductivity decreases.
— Ionic conduction. It exists in gaseous and liquid media, where there are free ions, which also carry charges, moving through the volume of the medium under the influence of an electromagnetic field or other external influence. Used in electrolytes. As the temperature rises, the ionic conductivity increases, since more high-energy ions are formed, and the viscosity of the medium also decreases.
— hole conduction. This conductivity is due to the lack of electrons in the crystal lattice of the material. In fact, here again, electrons transfer charge, but they seem to move along the lattice, occupying successively free places in it, in contrast to the physical movement of electrons in metals. This principle is used in semiconductors, along with electronic conduction.

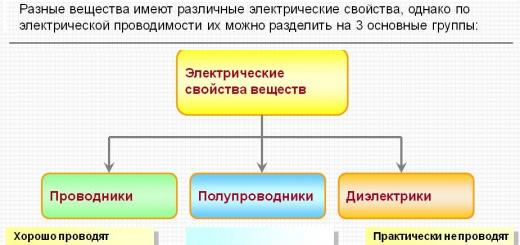
The very first materials that began to be used in electrical engineering historically were metals and dielectrics (insulators that have low electrical conductivity). Semiconductors are now widely used in electronics. They occupy an intermediate position between conductors and dielectrics and are characterized by the fact that the magnitude of electrical conductivity in semiconductors can be controlled by various influences. Silicon, germanium and carbon are used to manufacture most modern conductors. In addition, other substances can be used to make PP, but they are used much less frequently.
It is important to transfer current with minimal losses. In this regard, metals with high electrical conductivity and, accordingly, low electrical resistance play an important role. The best in this respect is silver (62500000 Sm/m), followed by copper (58100000 Sm/m), gold (45500000 Sm/m), aluminum (37000000 Sm/m). In accordance with economic feasibility, aluminum and copper are most often used, while copper is very slightly inferior in conductivity to silver. All other metals are not of industrial importance for the production of conductors.
In order to talk about electrical conductivity, you need to remember the nature of the electric current as such. So, when a substance is placed inside an electric field, charges move. This movement provokes the action of just an electric field. It is the flow of electrons that is the electric current. The strength of the current, as we know from school lessons in physics, is measured in Amperes and is denoted by the Latin letter I. 1 A is an electric current at which a charge of 1 Coulomb passes in a time equal to one second.
There are several types of electric current, namely:
- direct current, which does not change in relation to the indicator and the trajectory of movement at any time;
- alternating current, which changes its rate and trajectory over time (produced by generators and transformers);
- the pulsating current undergoes changes in magnitude, but does not change its direction.
The electrical conductivity index is directly related to the content of freely moving charges in the material, which have no connection with the crystal network, molecules or atoms.
Thus, according to the degree of current conductivity, materials are divided into the following types:
- conductors;
- dielectrics;
- semiconductors.
High electrical conductivity is treated in electronic theory. So, electrons run among atoms throughout the conductor due to their weak valence bond with the nuclei. That is, freely moving charged particles inside the metal close the voids among the atoms and are characterized by the randomness of movement. If, on the other hand, a metal conductor is placed in an electric field, the electrons will take order in their movement, moving to a pole with a positive charge. This is what creates the electric current. The speed of propagation of an electric field in space is similar to the speed of light. It is with this speed that the electric current moves inside the conductor. It is worth noting that this is not the speed of movement of the electrons themselves (their speed is very small and equals a maximum of several mm / s), but the speed of propagation of electricity throughout the substance.
With the free movement of charges inside the conductor, they meet various microparticles on their way, with which a collision occurs and some energy is given to them. Conductors are known to experience heat. This is just due to the fact that overcoming the resistance, the energy of the electrons is distributed as a heat release.
Such "accidents" of charges create an obstacle to the movement of electrons, which is called resistance in physics. A small resistance slightly heats the conductor, and at a high resistance, high temperatures are reached. The latter phenomenon is used in heating devices as well as traditional incandescent lamps. Resistance is measured in ohms. Designated with the Latin letter R.
Electrical conductivity- a phenomenon that reflects the ability of a metal or electrolyte to conduct an electric current. This value is the reciprocal of the electrical resistance.
The electrical conductivity is measured by Siemens (Cm), and is denoted by the letter G.
Since atoms create an obstacle to the passage of current, the resistance index of substances is different. For designation, the concept of resistivity (Ohm-m) was introduced, which just gives information about the conduction abilities of substances.
Modern conductive materials are in the form of thin ribbons, wires with a specific cross-sectional area and a certain length. Electrical conductivity and resistivity are measured in the following units: Sm-m/mm.kv and Ohm-mm.kv/m, respectively.
Thus, electrical resistivity and electrical conductivity are characteristics of the conductive capacity of a material, the cross-sectional area of \u200b\u200bwhich is 1 mm2 and length 1 m. The temperature for the characteristic is 20 degrees Celsius.
Good conductors of electric current among metals are precious metals, namely gold and silver, as well as copper, chromium and aluminum. Steel and iron conductors have weaker characteristics. It should be noted that pure metals have better electrical conductive properties compared to metal alloys. For high resistance, if necessary, tungsten, nichrome and constant conductors are used.
With knowledge of the indicators of resistivity or conductivity, it is very easy to calculate the resistance and electrical conductivity of a particular conductor. In this case, the length and cross-sectional area of a particular conductor must be used in the calculations.
It is important to know that the electrical conductivity index, as well as the resistance of any material, directly depends on the temperature regime. This is explained by the fact that with a change in temperature, there are also changes in the frequency and amplitude of atomic vibrations. Thus, with an increase in temperature, the resistance to the flow of moving charges will increase in parallel. And as the temperature decreases, the resistance decreases, and the electrical conductivity increases.
In some materials, the dependence of temperature on resistance is very pronounced, in some it is more weak.
electrical conductivity(electrical conductivity, conductivity) - the ability of a body to conduct electric current, as well as a physical quantity that characterizes this ability and is inverse to electrical resistance. In the International System of Units (SI), the unit of measurement of electrical conductivity is Siemens (Russian designation: Cm; international: S), defined as 1 Sm \u003d 1 Ohm -1, that is, as the electrical conductivity of a section of an electrical circuit with a resistance of 1 Ohm.
Encyclopedic YouTube
-
1 / 5
Specific conductivity (electrical conductivity) is a measure of the ability of a substance to conduct electric current. According to Ohm's law, in a linear isotropic substance, specific conductivity is a coefficient of proportionality between the density of the emerging current and the magnitude of the electric field in the medium:
J → = σ E → , (\displaystyle (\vec (J))=\sigma \,(\vec (E)))In an inhomogeneous medium, σ may depend (and generally depends) on the coordinates, that is, it does not coincide at different points of the conductor.
The specific conductivity of anisotropic (unlike isotropic) media is, generally speaking, not a scalar, but a tensor (symmetric tensor of rank 2), and multiplication by it reduces to matrix multiplication:
J i = ∑ k = 1 3 σ i k E k , (\displaystyle J_(i)=\sum \limits _(k=1)^(3)\sigma _(ik)\,E_(k),)in this case, the current density and field strength vectors are generally not collinear.
For any linear medium, one can choose locally (and if the medium is homogeneous, then globally) the so-called its own basis - an orthogonal system of Cartesian coordinates, in which the matrix becomes diagonal, that is, it takes on the form in which out of nine components i k (\displaystyle \sigma _(ik)) only three are different from zero: σ 11 (\displaystyle \sigma _(11)), σ 22 (\displaystyle \sigma _(22)) and σ 33 (\displaystyle \sigma _(33)). In this case, denoting σ i i (\displaystyle \sigma _(ii)) as , instead of the previous formula, we get a simpler one
J i = σ i E i . (\displaystyle J_(i)=\sigma _(i)E_(i).)Quantities i (\displaystyle \sigma _(i)) called principal values conductivity tensor. In the general case, the above relation is valid only in one coordinate system.
The reciprocal of conductivity is called resistivity.
Generally speaking, the linear relation written above (both scalar and tensor) is true at best approximately, and this approximation is good only for relatively small quantities E. However, even with these values E, when deviations from linearity are noticeable, the electrical conductivity can retain its role as a factor in the linear expansion term, while other higher expansion terms will give corrections that provide good accuracy. In the case of a non-linear dependence J from E introduced differential electrical conductivity σ = dJ / dE (\displaystyle \sigma =dJ/dE)(for anisotropic media: σ i k = d J i / d E k (\displaystyle \sigma _(ik)=dJ_(i)/dE_(k))).
electrical conductivity G conductor length L with cross-sectional area S can be expressed in terms of the specific conductivity of the substance from which the conductor is made, by the following formula:
G = σ S L . (\displaystyle G=\sigma (\frac (S)(L)).)Specific conductivity of some substances
Conductivity given at +20 °C :
substance cm/m silver 62 500 000 copper 59 500 000 gold 45 500 000 aluminum 38 000 000 magnesium 22 700 000 iridium 21 100 000 molybdenum 18 500 000 tungsten 18 200 000 zinc 16 900 000 nickel 11 500 000 pure iron 10 000 000 platinum 9 350 000 tin 8 330 000 cast steel 7 690 000 lead 4 810 000 nickel silver 3 030 000 constantan 2 000 000 manganin 2 330 000 1 040 000 nichrome 893 000 graphite 125 000 sea water 3 the ground is wet 10 −2 water distill. 10 −4 marble 10 −8 glass 10 −11 porcelain 10 −14 quartz glass 10 −16 amber 10 −18 Electrical conductivity of solutions
The speed of movement of ions depends on the strength of the electric field, temperature, viscosity of the solution, radius and charge of the ion, and interionic interaction.
In solutions of strong electrolytes, the nature of the concentration dependence of electrical conductivity is observed due to the action of two mutually opposite effects. On the one hand, as the dilution increases, the number of ions per unit volume of the solution decreases. On the other hand, their speed increases due to the weakening of braking by ions of the opposite sign.
The physical nature of electrical resistance. When free electrons move in a conductor, they collide on their way with positive ions 2 (see Fig. 10, a), atoms and molecules of the substance from which the conductor is made, and transfer part of their energy to them. In this case, the energy of moving electrons as a result of their collision with atoms and molecules is partially released and dissipated in the form of heat that heats the conductor. In view of the fact that electrons, colliding with particles of a conductor, overcome some resistance to movement, it is customary to say that conductors have electrical resistance. If the resistance of the conductor is small, it is relatively weakly heated by the current; if the resistance is high, the conductor may become hot. The wires that supply electric current to the electric stove almost do not heat up, since their resistance is small, and the spiral of the tile, which has high resistance, is red-hot. The filament of the electric lamp heats up even more.
The ohm is taken as the unit of resistance. A conductor has a resistance of 1 ohm, through which a current of 1 A passes with a potential difference at its ends (voltage) equal to 1 V. The standard of resistance of 1 ohm is a column of mercury 106.3 cm long and with a cross-sectional area of 1 mm2 at a temperature of 0 ° C. In practice, resistance is often measured in thousands of ohms - kiloohms (kOhm) or millions of ohms - megaohms (MΩ). Resistance is denoted by the letter R (r).
Conductivity. Any conductor can be characterized not only by its resistance, but also by the so-called conductivity - the ability to conduct electric current. Conductivity is the reciprocal of resistance. The unit of conductivity is called the Siemens (Sm). 1 cm is equal to 1/1 ohm. Conductivity is denoted by the letter G (g). Hence,G=1/R(4)
Specific electrical resistance and conductivity. Atoms of different substances have different resistance to the passage of electric current. The ability of individual substances to conduct electric current can be judged by their electrical resistivity p. The value characterizing the resistivity is usually taken as the resistance of a cube with an edge of 1 m. The electrical resistivity is measured in Ohm * m. To judge the electrical conductivity of materials, they also use the concept of specific electrical conductivity? = 1 /?. Electrical conductivity is measured in siemens per meter (S/m) (conductivity of a cube with a 1m edge). Often electrical resistivity is expressed in ohm-centimetres (Ohm*cm) and electrical conductivity in siemens per centimeter (S/cm). Wherein 1 Ohm * cm \u003d 10 -2 Ohm * m, and 1 S / cm \u003d 10 2 S / m.
Conducting materials are used mainly in the form of wires, tires or tapes, the cross-sectional area of \u200b\u200bwhich is usually expressed in square millimeters, and the length in meters. Therefore, for the specific electrical resistance of similar materials and specific electrical conductivity, other units of measurement are also introduced: measured in Ohm * mm 2 / m (resistance of a conductor 1 m long and 1 mm 2 cross-sectional area), eh? - in Sm * m / mm 2 (conductivity of a conductor 1 m long and with a cross-sectional area of \u200b\u200b1 mm 2).
Of the metals, silver and copper have the highest electrical conductivity, since the structure of their atoms allows free electrons to move easily, followed by gold, chromium, aluminum, manganese, tungsten, etc. Iron and steel conduct current worse.
Pure metals always conduct electricity better than their alloys. Therefore, in electrical engineering, very pure copper is mainly used, containing only 0.05% impurities. And vice versa, in cases where a material with high resistance is needed (for various heating devices, rheostats, etc.), special alloys are used: constantan, manganin, nichrome, fechral.
It should be noted that in technology, in addition to metallic conductors, non-metallic ones are also used. Such conductors include, for example, coal, from which the brushes of electrical machines are made, electrodes for searchlights, etc. Conductors of electric current are the thickness of the earth, living tissues of plants, animals and humans. Raw wood and many other insulating materials conduct electricity when wet.
The electrical resistance of a conductor depends not only on the material of the conductor, but also on its length l and cross-sectional area s. (Electrical resistance is similar to the resistance to the movement of water in a pipe, depending on the cross-sectional area of the pipe and its length.)
Straight conductor resistanceR= ? l/s (5)
If resistivity? expressed in Ohm * mm / m, then in order to obtain the resistance of the conductor in ohms, its length must be substituted into formula (5) in meters, and the cross-sectional area in square millimeters.
Dependence of resistance on temperature. The electrical conductivity of all materials depends on their temperature. In metal conductors, when heated, the range and speed of vibrations of atoms in the crystal lattice of the metal increase, as a result of which the resistance that they provide to the flow of electrons also increases. When cooled, the opposite phenomenon occurs: the random oscillatory motion of atoms in the nodes of the crystal lattice decreases, their resistance to the flow of electrons decreases, and the electrical conductivity of the conductor increases.
In nature, however, there are some alloys: fechral, constantan, manganin, and others, in which, in a certain temperature range, the electrical resistance changes relatively little. Such alloys are used in engineering for the manufacture of various resistors used in electrical measuring instruments and some devices to compensate for the effect of temperature on their operation.
The degree of change in the resistance of conductors with a change in temperature is judged by the so-called temperature coefficient of resistance a. This coefficient represents the relative increase in the resistance of the conductor with an increase in its temperature by 1 ° C. In table. 1 shows the values of the temperature coefficient of resistance for the most used conductor materials.
Resistance of a metal conductor R t at any temperature t
R t = R 0 [ 1 + ? (t - t 0) ] (6)
where R 0 is the resistance of the conductor at a certain initial temperature t 0 (usually at + 20 ° C), which can be calculated using formula (5);
t- t 0 - temperature change.
The property of metallic conductors to increase their resistance when heated is often used in modern technology to measure temperature. For example, when testing traction motors after repair, the heating temperature of their windings is determined by measuring their resistance in a cold state and after operating under load for a specified period (usually within 1 hour).
Exploring the properties of metals during deep (very strong) cooling, scientists discovered a remarkable phenomenon: near absolute zero (-273.16 ° C), some metals almost completely lose their electrical resistance. They become ideal conductors capable of passing current through a closed circuit for a long time without any influence from a source of electrical energy. This phenomenon is called superconductivity. At present, prototypes of power lines and electrical machines have been created that use the phenomenon of superconductivity. Such machines have significantly lower weight and overall dimensions compared to general purpose machines and operate with a very high efficiency. Power lines in this case can be made of wires with a very small cross-sectional area. In the future, this phenomenon will be used more and more in electrical engineering.
electrical conductivity System SI Type derivative Siemens(Russian designation: Cm; international designation: S) is a unit of electrical conductivity in the International System of Units (SI), the reciprocal of an ohm. By definition, Siemens is equal to the electrical conductivity of a conductor (section of an electrical circuit), the resistance of which is 1 ohm.
In terms of other SI units, Siemens is expressed as follows:
1 cm \u003d 1 / ohm \u003d / \u003d kg −1 −2 ³ ².
In accordance with the SI rules regarding derived units named after scientists, the name of the Siemens unit is written with a lowercase letter, and its designation with a capital letter.
Previously used name mo(eng. mho), which is the word "om" (ohm) read back; denoted by an inverted letter Ω: ℧ (\displaystyle \mho )(in Unicode U+2127 , ℧).
Before World War II (in the USSR until the 1960s), the Siemens was a unit of electrical resistance, corresponding to the resistance of a mercury column 1 m long and 1 mm in diameter at 0 °C. It corresponds to approximately 0.9534 ohms. This unit was introduced by Siemens in 1860 and competed with the ohm, which was finally chosen as the unit of resistance at the World Congress of Electrical Engineers in 1881. Nevertheless, Siemens as a unit of resistance was widely used by signalmen around the world until the middle of the 20th century.
Multiples and submultiples
Decimal multiples and submultiples are formed using standard SI prefixes.
Multiples Dolnye magnitude title designation magnitude title designation 10 1 cm decasimens yesSm daS 10 −1 cm decisiemens dSm dS 10 2 cm hectosiemens gsm hS 10 −2 cm centi-siemens ccm cS 10 3 cm kilosiemens kSm kS 10 −3 cm millisiemens mSm MS 10 6 cm megasiemens MSm MS 10 −6 cm microsiemens µS µS 10 9 cm gigasiemens GSM GS 10 −9 cm nanosense nS nS 10 12 cm terasiemens TSm TS 10 −12 cm picosiemens pSm PS 10 15 cm petasiemens PSm PS 10 −15 cm femtosiemens fsm fS 10 18 cm exasiemens esm ES 10 −18 cm attosiemens acm aS 10 21 cm zettasiemens ZSm ZS 10 −21 cm zeptosiemens zSm zS 10 24 cm yottasiemens ISM YS 10 −24 cm joctosiemens iSm yS apply