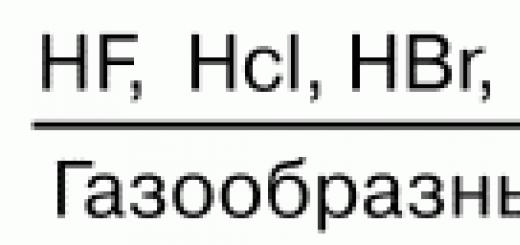Goals and objectives of the lesson: improving the skills of graphical problem solving, repetition of basic physical concepts on this topic; development of oral and written speech, logical thinking; activation of cognitive activity through the content and degree of complexity of tasks; generating interest in the topic.
Lesson plan.
During the classes
Necessary equipment and materials: computer, projector, screen, whiteboard, Ms Power Point program, for each student : laboratory thermometer, test tube with paraffin, test tube holder, glass with cold and hot water, calorimeter.
Control:
Start presentation "F5 key", stop - "Esc key".
Changes of all slides are organized by clicking the left mouse button (or by pressing the right arrow key).
Return to the previous slide "left arrow".
I. Repetition of the studied material.
1. What aggregate states of matter do you know? (Slide 1)
2. What determines this or that state of aggregation of a substance? (Slide 2)
3. Give examples of finding a substance in various states of aggregation in nature. (Slide 3)
4. What is the practical significance of the phenomena of the transition of matter from one state of aggregation to another? (Slide 4)
5. What process corresponds to the transition of a substance from a liquid state to a solid state? (Slide 5)
6. What process corresponds to the transition of a substance from a solid state to a liquid? (Slide 6)
7. What is sublimation? Give examples. (Slide 7)
8. How does the speed of the molecules of a substance change during the transition from a liquid to a solid state?
II. Learning new material
In the lesson, we will study the process of melting and crystallization of a crystalline substance - paraffin, and plot these processes.
In the course of performing a physical experiment, we will find out how the temperature of paraffin changes during heating and cooling.
You will perform the experiment according to the descriptions for the work.
Before starting work, I will remind you of the safety rules:
When performing laboratory work, be careful and careful.
Safety engineering.
1. Calorimeters contain water 60? C, be careful.
2. Use caution when handling glassware.
3. If the device is accidentally broken, then inform the teacher, do not remove the fragments yourself.
III. Frontal physical experiment.
On the tables of the students there are sheets with a description of the work (Appendix 2), according to which they perform the experiment, build a graph of the process and draw conclusions. (Slides 5).
IV. Consolidation of the studied material.
Summing up the results of the frontal experiment.
Findings:
When heated paraffin in the solid state to a temperature of 50? C, the temperature increases.
During melting, the temperature remains constant.
When all the paraffin has melted, the temperature increases with further heating.
When liquid paraffin is cooled, the temperature decreases.
During crystallization, the temperature remains constant.
When all of the paraffin has solidified, the temperature decreases with further cooling.
Structural diagram: "Melting and solidification of crystalline bodies"
(Slide 12) Work according to the scheme.
| Phenomena | Scientific facts | Hypothesis | Ideal object | Quantities | The laws | Application |
| When a crystalline body melts, the temperature does not change. When a crystalline solid solidifies, the temperature does not change. |
When a crystalline body melts, the kinetic energy of atoms increases, the crystal lattice is destroyed. During solidification, the kinetic energy decreases and the crystal lattice is built. |
A solid body is a body whose atoms are material points arranged in an orderly manner (crystal lattice), interacting with each other by forces of mutual attraction and repulsion. | Q is the amount of heat Specific heat of fusion |
Q = m - absorbed Q = m - stands out |
1. To calculate the amount of heat 2. For use in engineering, metallurgy. 3. thermal processes in nature (melting of glaciers, freezing of rivers in winter, etc.) 4. Write your examples. |
The temperature at which a solid changes to a liquid state is called the melting point.
The crystallization process will also proceed at a constant temperature. It is called the crystallization temperature. In this case, the melting temperature is equal to the crystallization temperature.
Thus, melting and crystallization are two symmetrical processes. In the first case, the substance absorbs energy from the outside, and in the second - it gives it to the environment.
Different melting temperatures determine the scope of various solids in everyday life and technology. Refractory metals are used to make heat-resistant structures in aircraft and rockets, nuclear reactors and electrical engineering.
Consolidation of knowledge and preparation for independent work.
1. The figure shows a graph of heating and melting of a crystalline body. (Slide)
2. For each of the situations listed below, select a graph that most accurately reflects the processes occurring with the substance:
a) copper is heated and melted;
b) zinc is heated to 400°C;
c) melting stearin is heated to 100°C;
d) iron taken at 1539°C is heated to 1600°C;
e) tin is heated from 100 to 232°C;
f) aluminum is heated from 500 to 700°C.
Answers: 1-b; 2-a; 3-in; 4-in; 5 B; 6-d;
The graph reflects observations of the change in temperature of two
crystalline substances. Answer the questions:
(a) At what time did the observation of each substance begin? How long did it last?
b) Which substance began to melt first? Which substance melted first?
c) State the melting point of each substance. Name the substances whose heating and melting graphs are shown.
4. Is it possible to melt iron in an aluminum spoon?
5.. Is it possible to use a mercury thermometer at the Pole of Cold, where the lowest temperature was recorded - 88 degrees Celsius?
6. The combustion temperature of powder gases is about 3500 degrees Celsius. Why doesn't the barrel of a gun melt when fired?
Answers: It is impossible, since the melting point of iron is much higher than the melting point of aluminum.
5. It is impossible, since mercury will freeze at this temperature, and the thermometer will fail.
6. It takes time to heat and melt a substance, and the short duration of the combustion of gunpowder does not allow the gun barrel to heat up to the melting point.
4. Independent work. (Appendix 3).
Option 1
Figure 1a shows a graph of heating and melting of a crystalline body.
I. What was the body temperature at the first observation?
1. 300 °C; 2. 600 °C; 3. 100 °C; 4. 50 °C; 5. 550 °C.
II. Which process on the graph characterizes segment AB?
III. What process on the graph characterizes the BV segment?
1. Heating. 2. Cooling. 3. Melting. 4. Curing.
IV. At what temperature did the melting process begin?
1. 50 °C; 2. 100 °C; 3. 600 °C; 4. 1200 °C; 5. 1000 °C.
V. How long did the body melt?
1. 8 min; 2. 4 min; 3. 12 min; 4. 16 min; 5.7 min.
VI. Did body temperature change during melting?
VII. What process on the graph characterizes the segment VG?
1. Heating. 2. Cooling. 3. Melting. 4. Curing.
VIII. What temperature was the body at the last observation?
1. 50 °C; 2. 500 °C; 3. 550 °С; 4. 40 °C; 5. 1100 °C.
Option 2
Figure 101.6 shows a graph of cooling and solidification of a crystalline body.
I. What temperature was the body at the first observation?
1. 400 °C; 2. 110°C; 3. 100 °C; 4. 50 °C; 5. 440 °C.
II. Which process on the graph characterizes segment AB?
1. Heating. 2. Cooling. 3. Melting. 4. Curing.
III. What process on the graph characterizes the BV segment?
1. Heating. 2. Cooling. 3. Melting. 4. Curing.
IV. At what temperature did the curing process begin?
1. 80 °C; 2. 350 °C; 3. 320 °С; 4. 450 °C; 5. 1000 °C.
V. How long did the body harden?
1. 8 min; 2. 4 min; 3. 12 min;-4. 16 min; 5.7 min.
VI. Did body temperature change during hardening?
1. Increased. 2. Decreased. 3. Has not changed.
VII. What process on the graph characterizes the segment VG?
1. Heating. 2. Cooling. 3. Melting. 4. Curing.
VIII. What temperature was the body at the time of the last observation?
1. 10 °C; 2. 500 °C; 3. 350 °C; 4. 40 °C; 5. 1100 °C.
Summing up the results of independent work.
1 option
I-4, II-1, III-3, IV-5, V-2, VI-3, VII-1, VIII-5.
Option 2
I-2, II-2, III-4, IV-1, V-2, VI-3, VII-2, VIII-4.
Additional material: Watch the video: "Ice melting at t<0C?"
Student reports on the use of melting and crystallization in industry.
Homework.
14 textbooks; questions and tasks for the paragraph.
Tasks and exercises.
Collection of problems by V. I. Lukashik, E. V. Ivanova, No. 1055-1057
Bibliography:
- Peryshkin A.V. Physics grade 8. - M.: Bustard. 2009.
- Kabardin O. F. Kabardina S. I. Orlov V. A. Tasks for the final control of students' knowledge in physics 7-11. - M.: Enlightenment 1995.
- Lukashik V. I. Ivanova E. V. Collection of problems in physics. 7-9. - M.: Enlightenment 2005.
- Burov V. A. Kabanov S. F. Sviridov V. I. Frontal experimental tasks in physics.
- Postnikov AV Checking students' knowledge in physics 6-7. - M.: Enlightenment 1986.
- Kabardin OF, Shefer NI Determination of solidification temperature and specific heat of paraffin crystallization. Physics at school No. 5 1993.
- Video cassette "School physical experiment"
- Pictures from sites.
To effectively plan all construction work, you need to know how long concrete hardens. And here there are a number of subtleties that largely determine the quality of the erected structure. Below we will describe in detail how the solution is dried, and what you need to pay attention to when organizing related operations.
To make the material reliable, it is important to properly organize its drying.
Theory of cement mortar polymerization
To manage the process, it is very important to understand exactly how it happens. That is why it is worthwhile to study in advance what constitutes the solidification of cement (find out here how to make flowerpots from concrete).
In fact, this process is multi-stage. It includes both a set of strength and the actual drying.
Let's look at these stages in more detail:
- The hardening of concrete and other cement-based mortars begins with the so-called setting. At the same time, the substance in the formwork enters into a primary reaction with water, due to which it begins to acquire a certain structure and mechanical strength.
- Setting time depends on many factors. If we take the air temperature of 200C as a standard, then for the M200 solution the process starts approximately two hours after pouring and lasts about an hour and a half.
- After curing, the concrete hardens. Here, the bulk of the cement granules react with water (for this reason, the process is sometimes called cement hydration). The optimal conditions for hydration are air humidity of about 75% and temperature from 15 to 200C.
- At temperatures below 100C, there is a risk that the material will not gain design strength, which is why special anti-frost additives must be used to work in the winter.

Curing chart
- The strength of the finished structure and the rate of curing of the solution are interrelated. If the composition loses water too quickly, then not all the cement will have time to react, and low density pockets will form inside the structure, which can become a source of cracks and other defects.
Note! Cutting reinforced concrete with diamond wheels after polymerization often clearly demonstrates the inhomogeneous structure of slabs poured and dried in violation of technology.

Photo cut with clearly visible defects
- Ideally, the mortar needs 28 days to fully cure.. However, if too strict requirements for bearing capacity are not put forward to the structure, then it can be started to operate already three to four days after pouring.
When planning construction or repair work, it is important to correctly evaluate all the factors that will affect the rate of dehydration of the solution (see also the article “Non-autoclaved aerated concrete and its features”).
Experts highlight the following points:

Vibrocompaction process
- First, environmental conditions play an important role. Depending on the temperature and humidity, the poured foundation can either dry out in just a few days (and then it will not gain design strength), or remain wet for more than a month.
- Secondly, the packing density. The denser the material, the slower it loses moisture, which means that the cement is hydrated more efficiently. For compaction, vibration processing is most often used, but when doing work with your own hands, you can get by with bayoneting.
Advice! The denser the material, the more difficult it is to process after hardening. That is why for structures, during the construction of which vibration compaction was used, diamond drilling of holes in concrete is most often required: conventional drills wear out too quickly.
- The composition of the material also affects the speed of the process. The rate of dehydration mainly depends on the porosity of the filler: expanded clay and slag accumulate microscopic moisture particles and release them much more slowly than sand or gravel.
- Also, water-retaining additives (bentonite, soap solutions, etc.) are widely used to slow down drying and more effective curing. Of course, the price of the structure increases, but there is no need to worry about premature drying.

Modifier for concrete
- In addition to all of the above, the instruction recommends paying attention to the formwork material. Porous walls made of unedged boards draw a significant amount of liquid from the edge sections. Therefore, to ensure strength, it is better to use formwork made of metal shields or to lay a plastic film inside a wooden box.

Porous formwork actively "draws" moisture from the material
Tips for organizing the process
Self-pouring of concrete foundations and floors should be carried out according to a certain algorithm.
To keep moisture in the thickness of the material and contribute to the maximum set of strength, you need to act like this:
- To begin with, we carry out high-quality waterproofing of the formwork. To do this, we cover the wooden walls with polyethylene or use special plastic collapsible shields.
- We introduce modifiers into the composition of the solution, the action of which is aimed at reducing the rate of evaporation of the liquid. You can also use additives that allow the material to gain strength faster, but they are quite expensive, and therefore they are used mainly in multi-storey construction.
- Then we pour concrete, carefully compacting it. For this purpose, it is best to use a special vibration tool. If there is no such device, we process the poured mass with a shovel or a metal rod, removing air bubbles.

The less moisture leaves in the first days, the stronger the base will be.
- The surface of the solution after setting is covered with a plastic film. This is done in order to reduce moisture loss in the first few days after laying.
Note! In autumn, the polyethylene also protects the outdoor cement from precipitation that erodes the surface layer.
- After about 7-10 days, the formwork can be dismantled. After dismantling, we carefully examine the walls of the structure: if they are wet, then you can leave them open, but it is better to cover the dry ones with polyethylene too.
- After that, every two or three days we remove the film and inspect the surface of the concrete. If a large amount of dust, cracks or delamination of the material appears, we moisten the hardened solution from the hose and cover it again with polyethylene.
- On the twentieth day, remove the film and continue drying in natural mode.
- After 28 days have passed from the moment of pouring, the next stage of work can begin. At the same time, if we did everything correctly, you can load the structure “to the fullest” - its strength will be maximum!
Knowing how long the concrete foundation hardens, we can properly organize all other construction work. However, this process cannot be accelerated, since cement only acquires the necessary performance characteristics when it hardens for a sufficient time (learn also how to build a concrete cellar).
For more information on this issue, see the video in this article.
To effectively plan all construction work, you need to know how long concrete hardens. And here there are a number of subtleties that largely determine the quality of the erected structure. Below we will describe in detail how the solution is dried, and what you need to pay attention to when organizing related operations.
Theory of cement mortar polymerization
To manage the process, it is very important to understand exactly how it happens. That is why it is worthwhile to study in advance what constitutes the solidification of cement ().
In fact, this process is multi-stage. It includes both a set of strength and the actual drying.
Let's look at these stages in more detail:
- The hardening of concrete and other cement-based mortars begins with the so-called setting. At the same time, the substance in the formwork enters into a primary reaction with water, due to which it begins to acquire a certain structure and mechanical strength.
- Setting time depends on many factors. If we take the air temperature of 20 0 С as a standard, then for the M200 solution the process starts approximately two hours after pouring and lasts about an hour and a half.
- After curing, the concrete hardens. Here, the bulk of the cement granules react with water (for this reason, the process is sometimes called cement hydration). The optimal conditions for hydration are air humidity of about 75% and temperature from 15 to 20 0 C.
- At temperatures below 10 0 C, there is a risk that the material will not gain design strength, which is why special anti-frost additives must be used to work in the winter.

- The strength of the finished structure and the rate of curing of the solution are interrelated. If the composition loses water too quickly, then not all the cement will have time to react, and low density pockets will form inside the structure, which can become a source of cracks and other defects.
Note! Cutting reinforced concrete with diamond wheels after polymerization often clearly demonstrates the inhomogeneous structure of slabs poured and dried in violation of technology.

- Ideally, the mortar needs 28 days to fully cure.. However, if too strict requirements for bearing capacity are not put forward to the structure, then it can be started to operate already three to four days after pouring.
Factors affecting freezing
When planning construction or repair work, it is important to correctly evaluate all the factors that will affect the rate of dehydration of the solution ().
Experts highlight the following points:

- First, environmental conditions play an important role. Depending on the temperature and humidity, the poured foundation can either dry out in just a few days (and then it will not gain design strength), or remain wet for more than a month.
- Secondly, the packing density. The denser the material, the slower it loses moisture, which means that the cement is hydrated more efficiently. For compaction, vibration processing is most often used, but when doing work with your own hands, you can get by with bayoneting.
Advice! The denser the material, the more difficult it is to process after hardening. That is why for structures, during the construction of which vibration compaction was used, diamond drilling of holes in concrete is most often required: conventional drills wear out too quickly.
- The composition of the material also affects the speed of the process. The rate of dehydration mainly depends on the porosity of the filler: expanded clay and slag accumulate microscopic moisture particles and release them much more slowly than sand or gravel.
- Also, water-retaining additives (bentonite, soap solutions, etc.) are widely used to slow down drying and more effective curing. Of course, the price of the structure increases, but there is no need to worry about premature drying.

- In addition to all of the above, the instruction recommends paying attention to the formwork material. Porous walls made of unedged boards draw a significant amount of liquid from the edge sections. Therefore, to ensure strength, it is better to use formwork made of metal shields or to lay a plastic film inside a wooden box.

Self-pouring of concrete foundations and floors should be carried out according to a certain algorithm.
To keep moisture in the thickness of the material and contribute to the maximum set of strength, you need to act like this:
- To begin with, we carry out high-quality waterproofing of the formwork. To do this, we cover the wooden walls with polyethylene or use special plastic collapsible shields.
- We introduce modifiers into the composition of the solution, the action of which is aimed at reducing the rate of evaporation of the liquid. You can also use additives that allow the material to gain strength faster, but they are quite expensive, and therefore they are used mainly in multi-storey construction.
- Then we pour concrete, carefully compacting it. For this purpose, it is best to use a special vibration tool. If there is no such device, we process the poured mass with a shovel or a metal rod, removing air bubbles.

- The surface of the solution after setting is covered with a plastic film. This is done in order to reduce moisture loss in the first few days after laying.
Note! In autumn, the polyethylene also protects the outdoor cement from precipitation that erodes the surface layer.
- After about 7-10 days, the formwork can be dismantled. After dismantling, we carefully examine the walls of the structure: if they are wet, then you can leave them open, but it is better to cover the dry ones with polyethylene too.
- After that, every two or three days we remove the film and inspect the surface of the concrete. If a large amount of dust, cracks or delamination of the material appears, we moisten the hardened solution from the hose and cover it again with polyethylene.
- On the twentieth day, remove the film and continue drying in natural mode.
- After 28 days have passed from the moment of pouring, the next stage of work can begin. At the same time, if we did everything correctly, you can load the structure “to the fullest” - its strength will be maximum!
Conclusion
Knowing how long the concrete foundation hardens, we can properly organize all other construction work. However, this process cannot be accelerated, since cement acquires the necessary performance characteristics only when it hardens for a sufficient time ().
For more information on this issue, see the video in this article.
Aggregate states of matter. Melting and solidification of crystalline bodies. Melting and hardening chart
Target: aggregate states of matter, location, nature of movement and interaction of molecules in different aggregate states, crystalline bodies, melting and solidification of crystalline bodies, melting temperature, graph of melting and solidification of crystalline bodies (using ice as an example)
Demos. 1. Model of the crystal lattice.
2. Melting and solidification of crystalline bodies (for example, ice).
3. Formation of crystals.
StageTime, min
Techniques and methods
1. Setting the objectives of the lesson. Introductory conversation.
2. Learning new material.
3.Fixing
material
4. Physical education minute
4. Checking the assimilation of the topic
4. Summing up
Teacher's message
Frontal conversation, demonstration experiment, group work, individual task
Group solution of qualitative and graphic tasks, frontal survey.
Testing
Grading, writing on the board and in diaries
1.Organization of the class
2. Studying the topic
I . Test questions:
What is the state of aggregation of matter?
Why is it necessary to study the transition of matter from one state of aggregation to another?
What is melting?
II . Explanation of the new material:
Comprehending the laws of nature and using them in their practical activities, a person becomes more and more powerful. Gone are the days of mystical fear of nature. Modern man is increasingly acquiring power over the forces of nature, is increasingly using these forces, the wealth of nature to accelerate scientific and technological progress.
Today we will comprehend new laws of nature, new concepts that will allow us to better know the world around us, and therefore use them correctly for the benefit of man.
I .Aggregate states of matter
Frontal discussion on:
What is a substance?
What do you know about matter?
Demonstration : crystal lattice models
What states of matter do you know?
Describe each state of matter.
Explain the properties of matter in solid, liquid, gaseous states.
Conclusion: a substance can be in three states - liquid, solid and gaseous, they are called aggregate states of matter.
II .Why is it necessary to study the aggregate states of matter
Amazing substance water
Water has many amazing properties that sharply distinguish it from all other liquids. And if the water behaved as expected, then the Earth would become simply unrecognizable
All bodies expand when heated and contract when cooled. Everything except water. At temperatures from 0 to + 4 0 Water expands when cooled and contracts when heated. At + 4 0 c water has the highest density, equal to 1000 kg / m 3 .At lower and higher temperatures, the density of water is somewhat less. Due to this, convection occurs in a peculiar way in autumn and winter in deep reservoirs. Water, cooling from above, sinks down to the bottom only until its temperature drops to + 4 0 C. Then the temperature distribution is established in a stagnant reservoir. To heat 1 g of water by 1 0 with it it is necessary to give 5, 10, 30 times more heat than 1 g of any other substance.
The anomaly of water - a deviation from the normal properties of bodies - is not fully understood, but their main reason is known: the structure of the water molecule. Hydrogen atoms attach to the oxygen atom not symmetrically from the sides, but gravitate to one side. Scientists believe that if not for this asymmetry, the properties of water would change dramatically. For example, water would solidify at -90 0 C and would boil at - 70 0 WITH.
III .Melting and solidification
Under blue skies
Magnificent carpets
Snow glittering in the sun
The transparent forest alone turns black
And the spruce turns green through the hoarfrost
And the river under the ice glitters
A.S. Pushkin
Inevitably it will snow
Like a pendulum's steady swing
Snow falls, swirls, curls
Lies evenly on the house
Stealthily penetrates into the bins
Flies into cars into pits and wells
E.Verharga
And I stroked the snow with my hand
And he shone with stars
There is no such sadness in the world
Which the snow wouldn't heal
He is like music. He is the message
His recklessness is boundless
Ah, this snow. ... No wonder it has
There is always some secret...
S.G.Ostrovoy
What substance are we talking about in these quatrains?
What state is the substance in?
V .Independent work of students in pairs
2. Study the table "Melting point of some substances"
3. Consider the graph in Figure 16
4. Interrogation in pairs (each pair is given questions on cards ):
What is melting?
What is the melting point?
What is called solidification or crystallization?
Which of the substances listed in the table has the highest melting point? What is its curing temperature?
Which of the substances listed in the table hardens at temperatures below 0 0 WITH?
At what temperature does alcohol solidify?
What happens to water in the segment AB, BC,CD, DE, TF, FK.
How can one judge the change in temperature of a substance during heating and cooling from the graph?
What parts of the graph correspond to the melting and solidification of ice?
Why are these sections parallel to the time axis?
VII. Demonstration: Melting and solidification of crystalline bodies (on the example of ice).
Phenomenon observation
VIII.Frontal conversation on the proposed issues.
Findings:
Melting is the transition of a substance from a solid to a liquid state;
Solidification or crystallization is the transition of a substance from liquid to solid.
The melting point is the temperature at which a substance melts.
A substance solidifies at the same temperature as it melts.
During the melting and solidification processes, the temperature does not change.
Physical education minute
Exercises to relieve fatigue from the shoulder girdle, arms and torso.
VII.Securing.
1. Solving quality problems
Why are thermometers with alcohol rather than mercury used to measure the outside air temperature in cold areas?
What metals can be melted in a copper pot?
What happens to tin if it is thrown into molten lead?
What happens to a piece of lead if it is thrown into liquid tin at its melting point?
What happens to mercury if it is poured into liquid nitrogen?
2.Solving graphic problems
Describe the processes occurring with the substance according to the graph below. What is this substance?
Describe the processes that occur with aluminum according to the graph below. Where does the decrease in the internal energy of a solid occur?
600
400
200
200
400
The figures show graphs of the dependence of temperature on time for two bodies of the same mass. Which substance has the highest melting point? Which body has the highest specific heat of fusion? Are the specific heat capacities of the bodies the same?
Page 152 "Entertaining Physics" Book 2, Perelman
IX.Checking the assimilation of the topic - test
1. Aggregate states of matter are different
A. Molecules that make up the substance
B. The arrangement of the molecules of a substance
B. The arrangement of molecules, the nature of movement and the interaction of molecules
2. The melting of a substance is
A. The transition of a substance from a liquid to a solid state
B. The transition of a substance from gaseous to liquid
B. The transition of a substance from a solid to a liquid state
3. The melting point is called
A. The temperature at which the substance melts
B. The temperature of the substance
B.Temperature above 100 0 With
4. During the melting process, the temperature
A. Remains constant
B. Increases
B. Decreases
5.In an aluminum spoon can be melted
A. Silver
B.Zinc
V.Med
On house. §12-14, exercise 7(3-5), repeat the answer plan about the physical phenomenon.