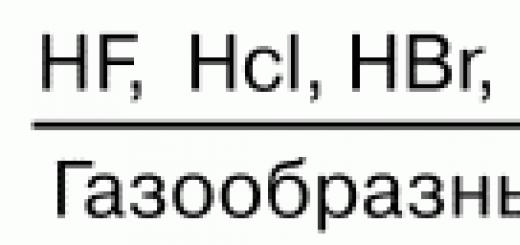Halogens- elements of group VII - fluorine, chlorine, bromine, iodine, astatine (astatine is little studied due to its radioactivity). Halogens are pronounced non-metals. Only iodine in rare cases exhibits some properties similar to metals.
In the unexcited state, halogen atoms have a common electronic configuration: ns2np5. This means that halogens have 7 valence electrons, except for fluorine.
Physical properties of halogens: F2 - colorless, difficult to liquefy gas; Cl2 is a yellow-green, easily liquefied gas with a sharp, suffocating odor; Br2 is a red-brown liquid; I2 is a purple crystalline substance.

Aqueous solutions of hydrogen halides form acids. HF - hydrofluoric (hydrofluoric); HCl - hydrochloric (hydrochloric); HBr - hydrogen bromide; HI - hydroiodine. The strength of acids decreases from top to bottom. Hydrofluoric acid is the weakest in the series of halogenated acids, and hydroiodic acid is the strongest. This is explained by the fact that the binding energy H2 decreases from above. In the same direction, the strength of the NH molecule also decreases, which is associated with an increase in the internuclear distance. The solubility of sparingly soluble salts in water also decreases:
From left to right, the solubility of halides decreases. AgF is highly soluble in water. All free halogens are oxidizing agents.. Their strength as oxidizing agents decreases from fluorine to iodine. In the crystalline, liquid and gaseous state, all halogens exist as individual molecules. The atomic radii increase in the same direction, which leads to an increase in the melting and boiling points. Fluorine dissociates into atoms better than iodine. The electrode potentials decrease when moving down the halogen subgroup. Fluorine has the highest electrode potential. Fluorine is the strongest oxidizing agent. Any higher free halogen will displace the lower one, which is in the state of a negative singly charged ion in solution.
20. Chlorine. Hydrogen chloride and hydrochloric acid
Chlorine (Cl) - stands in the 3rd period, in the VII group of the main subgroup of the periodic system, serial number 17, atomic mass 35.453; refers to halogens.
Physical properties: yellow-green gas with a pungent odor. Density 3.214 g/l; melting point -101 °C; boiling point -33.97 °C, At ordinary temperature, it is easily liquefied under a pressure of 0.6 MPa. Dissolving in water, it forms yellowish chlorine water. Let's well dissolve in organic solvents, especially in hexane (C6H14), in carbon tetrachloride.
Chemical properties of chlorine: electronic configuration: 1s22s22p63s22p5. There are 7 electrons in the outer level. Before the level is completed, 1 electron is needed, which chlorine accepts, showing an oxidation state of -1. There are also positive oxidation states of chlorine up to + 7. The following oxides of chlorine are known: Cl2O, ClO2, Cl2O6 and Cl2O7. All of them are unstable. Chlorine is a strong oxidizing agent. It directly reacts with metals and non-metals:
Reacts with hydrogen. Under normal conditions, the reaction proceeds slowly, with strong heating or lighting - with an explosion, according to a chain mechanism:
![]()
Chlorine interacts with alkali solutions, forming salts - hypochlorites and chlorides:
When chlorine is passed into an alkali solution, a mixture of chloride and hypochlorite solutions is formed:
Chlorine is a reducing agent: Cl2 + 3F2 = 2ClF3.
Interaction with water:
Chlorine does not interact directly with carbon, nitrogen and oxygen.
Receipt: 2NaCl + F2 = 2NaF + Cl2.
Electrolysis: 2NaCl + 2H2O = Cl2 + H2 + 2NaOH.
Finding in nature: contained in the composition of minerals: halite (rock salt), sylvin, bischofite; sea water contains chlorides of sodium, potassium, magnesium and other elements.
Hydrogen chloride HCl. Physical properties: colorless gas, heavier than air, soluble in water to form hydrochloric acid.
Receipt: in the laboratory:
In industry: they burn hydrogen in a stream of chlorine. Next, hydrogen chloride is dissolved in water, and hydrochloric acid is obtained (see above).
Chemical properties: hydrochloric acid - strong, monobasic, interacts with metals standing in a series of voltages up to hydrogen: Zn + 2HCl = ZnCl2 + H2.
As a reducing agent reacts with oxides and hydroxides of many metals.
DEFINITION
Halogens- elements of VII A group - fluorine (F), chlorine (Cl), bromine (Br) and iodine (I).
Electronic configuration of the external energy level of halogens ns 2 np 5 . Since, before the completion of the energy level, halogens lack only one electron, in OVR they most often exhibit the properties of oxidizing agents. Halogen oxidation states: from "-1" to "+7". The only element of the halogen group - fluorine - exhibits only one oxidation state "-1" and is the most electronegative element. Halogen molecules are diatomic: F 2 , Cl 2 , Br 2 , I 2 .
Chemical properties of halogens
With an increase in the charge of the nucleus of an atom of a chemical element, i.e. when moving from fluorine to iodine, the oxidizing ability of halogens decreases, which is confirmed by the ability to displace lower halogens by higher ones from hydrohalic acids and their salts:
Br 2 + 2HI = I 2 + 2HBr;
Cl 2 + 2KBr = Br 2 + 2KCl.
Fluorine has the highest chemical activity. Most chemical elements even at room temperature interact with fluorine, releasing a large amount of heat. Even water burns in fluorine:
2H 2 O + 2F 2 \u003d 4HF + O 2.
Free chlorine is less reactive than fluorine. It does not directly react with oxygen, nitrogen and noble gases. It interacts with all other substances like fluorine:
2Fe + Cl 2 = 2FeCl 3;
2P + 5Cl 2 = 2PCl 5 .
When chlorine interacts with water in the cold, a reversible reaction occurs:
Cl 2 + H 2 O↔HCl + HClO.
The mixture, which is the reaction products, is called chlorine water.
When chlorine interacts with alkalis in the cold, mixtures of chlorides and hypochlorites are formed:
Cl 2 + Ca (OH) 2 \u003d Ca (Cl) OCl + H 2 O.
When chlorine is dissolved in a hot alkali solution, the following reaction occurs:
3Cl 2 + 6KOH \u003d 5KCl + KClO 3 + 3H 2 O.
Bromine, like chlorine, dissolves in water and, partially reacting with it, forms the so-called "bromine water", while iodine is practically insoluble in water.
Iodine differs significantly in chemical activity from other halogens. It does not react with most non-metals, and reacts slowly with metals only when heated. The interaction of iodine with hydrogen occurs only with strong heating, the reaction is endothermic and highly reversible:
H 2 + I 2 \u003d 2HI - 53 kJ.
Physical properties of halogens
At n.o. Fluorine is a light yellow gas with a pungent odor. Poisonous. Chlorine is a light green gas, like fluorine, it has a pungent odor. Strongly poisonous. At elevated pressure and room temperature, it easily turns into a liquid state. Bromine is a heavy red-brown liquid with a characteristic unpleasant pungent odor. Liquid bromine, as well as its vapors, are highly toxic. Bromine is poorly soluble in water and readily soluble in non-polar solvents. Iodine is a dark gray solid with a metallic sheen. Vapors of iodine are purple in color. Iodine sublimes easily, i.e. transforms into a gaseous state from a solid, while bypassing the liquid state.
Obtaining halogens
Halogens can be obtained by electrolysis of solutions or melts of halides:
MgCl 2 = Mg + Cl 2 (melt).
Most often, halogens are obtained by the oxidation reaction of hydrohalic acids:
MnO 2 + 4HCl \u003d MnCl 2 + Cl 2 + 2H 2 O;
K 2 Cr 2 O 7 + 14HCl = 3Cl 2 + 2KCl + 2CrCl 3 + 7H 2 O;
2KMnO 4 + 16HCl \u003d 2MnCl 2 + 5Cl 2 + 8H 2 O + 2KCl.
Application of halogens
Halogens are used as raw materials for various products. Thus, fluorine and chlorine are used for the synthesis of various polymeric materials, chlorine is also a raw material in the production of hydrochloric acid. Bromine and iodine are widely used in medicine, bromine is also used in the paint industry.
Examples of problem solving
EXAMPLE 1
| Exercise | Calculate the volume of chlorine (n.a.) that reacted with potassium iodide if iodine was formed with a mass of 508 g | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Decision | Let us write the reaction equation for the interaction of chlorine with potassium iodide: Cl 2 + 2KI \u003d I 2 + 2KCl The molar mass of iodine, calculated using the table of chemical elements of D.I. Mendeleev, is equal to - 254 g / mol. Find the amount of substance formed iodine: v(I 2) = m(I 2)/M(I 2) At, opened in 1940
The orbital distribution of electrons in the outer electron layer is the same for all halogens
They have much in common in the structure of atoms and molecules. They are finishing building R-shells of the outer layer, so they all belong to the number of p-elements. The outer electron layer of halogen atoms lacks one electron to complete, therefore, the electronegativity of these elements is pronounced and in redox reactions they behave mainly as oxidizing agents. ■ 1. How does the value of the atomic radius change depending on the increase in the charge of the atomic nucleus?
Physical properties of halogensAll properties of halogens, both physical and chemical, depend on the structure of the atoms of the elements. These properties of various halogens are largely similar, but at the same time, each halogen has a number of features. ■ 7. How does the color intensity of halogens change with increasing nuclear charges?
10. Compile and fill in the table "Physical properties of halogens" according to the following model:
Physiological action of halogensAll are poisonous in their physiological action. Fluorine is especially poisonous: when inhaled in small quantities, it causes pulmonary edema, in large quantities it causes destruction of lung tissue and death. iodine the least toxic of all halogens. Inhalation of iodine vapor when it is heated can cause poisoning, but it is rare to work with vaporous iodine, for example, when cleaning it by sublimation. Crystalline iodine should not be taken by hand, as when it comes into contact with the skin, it causes the appearance of characteristic yellow spots. All work with halogens should be done in a fume hood. Write down safety precautions for working with halogens and first aid in case of poisoning in a notebook. Chemical properties of halogens
By the nature of their chemical properties, as noted above, all halogens are typical non-metals with significant electronegativity. The most electronegative element with the highest non-metallic activity is fluorine, the least active is iodine. Rice. 21. Combustion of hydrogen in chlorine. 1- chlorine 2- Interaction of halogens with simple substances. You can trace the decrease in chemical activity from fluorine to chlorine using examples of various reactions. Of particular interest is the interaction of various halogens with hydrogen. Their reaction conditions are different. Fluoride is the most durable compound among the hydrogen halides. With hydrogen, bromine forms hydrogen bromide.
Halogens also show oxidizing properties when interacting with metals, which usually proceeds very actively. Rice. 22. For example. Cu + Cl2 = CuCl2 Here, in reaction with chlorine, it exhibits an oxidation state equal to +3 - Fe +3, and equal to +2- Cu +2. In all the above cases, chlorine behaves like . Chemistry of the Elements Nonmetals of VIIA-subgroup Elements of the VIIA-subgroup are typical non-metals with a high electronegativity, they have a group name - "halogens". Key Issues Addressed in the Lecture General characteristics of non-metals of the VIIA-subgroup. Electronic structure, the most important characteristics of atoms. The most characteristic oxidation foam. Features of the chemistry of halogens. simple substances. natural compounds. Halogen compounds Hydrohalic acids and their salts. Salt and hydrofluoric acid slots, receiving and applying. halide complexes. Binary oxygen compounds of halogens. Instability ok- The redox properties of simple substances and co- unities. Disproportionation reactions. Latimer diagrams.
Chemistry of elements of the VIIA-subgroup general characteristics
VIIA group is formed by p-elements: fluorine F, chlorine Cl, bromine Br, iodine I and astatine At. The general formula for valence electrons is ns 2 np 5. All elements of group VIIA are typical non-metals.
to form a stable eight-electron lochki, so they have a strong tendency towards the addition of an electron. All elements easily form simple singly charged nye anions Г – . In the form of simple anions, elements of group VIIA are found in natural water and in crystals of natural salts, for example, halite NaCl, sylvin KCl, fluorite CaF2. Common group name of elements VIIA- groups "halogens", i.e. "giving birth to salts", due to the fact that most of their compounds with metals pre- is a typical salt (CaF2, NaCl, MgBr2, KI), which which can be obtained by direct mutual interaction of a metal with a halogen. Free halogens are obtained from natural salts, so the name "halogens" is also translated as "born from salts."
The minimum oxidation state (–1) is the most stable all halogens. Some characteristics of the atoms of the elements of group VIIA are given in The most important characteristics of atoms of elements of the VIIA group
Halogens have a high electron affinity (maximum for Cl) and a very high ionization energy (maximum for F) and maximum possible electronegativity in each of the periods. Fluorine is the most electronegative of all chemical elements. The presence of one unpaired electron in halogen atoms causes leads to the union of atoms in simple substances into diatomic molecules Г2. For simple halogen substances, oxidizing agents are most characteristic. properties that are strongest for F2 and weaken on passing to I2. Halogens are characterized by the greatest reactivity of all non-metallic elements. Fluorine, even among halogens, is isolated is extremely active. The element of the second period, fluorine, differs most strongly from the others. some elements of the subgroup. This is a general pattern for all non-metals.
Fluorine, as the most electronegative element, does not show gender living oxidation states. In any connections, including with Ki- oxygen, fluorine is in the oxidation state (-1). All other halogens exhibit positive oxidation states. up to a maximum of +7. The most characteristic oxidation states of halogens: F: -1, 0; Cl, Br, I: -1, 0, +1, +3, +5, +7. Oxides are known for Cl, in which it is in the oxidation states: +4 and +6. The most important halogen compounds, in positive oxidation foams are oxygen-containing acids and their salts. All halogen compounds in positive oxidation states are are strong oxidizing agents. terrible oxidation state. Disproportionation is promoted by an alkaline environment. Practical application of simple substances and oxygen compounds halogens is mainly due to their oxidizing effect. Simple substances Cl2 find the widest practical application. and F2. The largest amount of chlorine and fluorine is consumed in industrial or- ganic synthesis: in the production of plastics, refrigerants, solvents, pesticides, drugs. A significant amount of chlorine and iodine is used to obtain metals and for their refining. Chlorine is also used for bleaching cellulose, for the disinfection of drinking water and in the production of water of bleach and hydrochloric acid. Salts of oxo acids are used in the manufacture of explosives.
Acids are widely used in practice - hydrochloric and melting Fluorine and chlorine are among the twenty most common elements there, much less bromine and iodine in nature. All halogens are found in nature in the oxidation state(-one). Only iodine is found in the form of salt KIO3, which, as an impurity, is included in the Chilean saltpeter (KNO3). Astatine is an artificially obtained radioactive element (it does not exist in nature). The instability of At is reflected in the name, which comes from the Greek. "astatos" - "unstable". Astatine is a convenient emitter for radiotherapy of cancerous tumors. Simple substances Simple substances of halogens are formed by diatomic molecules G2. In simple substances, during the transition from F2 to I2 with an increase in the number of electrons electron layers and an increase in the polarizability of atoms, there is an increase intermolecular interaction, leading to a change in the aggregate standing under standard conditions. Fluorine (under normal conditions) is a yellow gas, at -181 ° C it turns into liquid state. Chlorine is a yellow-green gas, it turns into a liquid at -34 ° C. With a color of ha- the name Cl is associated with it, it comes from the Greek "chloros" - "yellow- green". A sharp increase in the boiling point of Cl2 compared to F2, indicates an increase in intermolecular interaction. Bromine is a dark red, very volatile liquid, boils at 58.8 ° C. On- the title of the element is associated with a sharp unpleasant smell of gas and is formed from "bromos" - "stinking". Iodine - dark purple crystals, with a slight "metallic" luster skom, which, when heated, easily sublimes, forming violet vapors;
the boiling point of iodine is 183o C. Its name comes from the color of iodine vapor - "iodos" - "violet". All simple substances have a pungent odor and are poisonous. Inhalation of their vapors causes irritation of the mucous membranes and respiratory organs, and at high concentrations - suffocation. During World War I, chlorine was used as a poison. Gaseous fluorine and liquid bromine cause skin burns. Working with ha- logens, precautions should be taken. Since the simple substances of halogens are formed by non-polar molecules cools, they dissolve well in non-polar organic solvents: alcohol, benzene, carbon tetrachloride, etc. In water, chlorine, bromine and iodine are sparingly soluble, their aqueous solutions are called chlorine, bromine and iodine water. Br2 dissolves better than others, the concentration of bromine in sat- brine solution reaches 0.2 mol/l, and chlorine - 0.1 mol/l. Fluorine decomposes water: 2F2 + 2H2O = O2 + 4HF Halogens exhibit high oxidative activity and transition dyat into halide anions. Г2 + 2e– 2Г– Fluorine has a particularly high oxidative activity. Fluorine oxidizes noble metals (Au, Pt). Pt + 3F2 = PtF6 It even interacts with some inert gases (krypton, xenon and radon), for example, Xe + 2F2 = XeF4 Many very stable compounds burn in an F2 atmosphere, for example, water, quartz (SiO2). SiO2 + 2F2 = SiF4 + O2
In reactions with fluorine, even such strong oxidizing agents as nitric and sulfur acid, act as reducing agents, while fluorine oxidizes included in their composition O(–2). 2HNO3 + 4F2 = 2NF3 + 2HF + 3O2 H2 SO4 + 4F2 = SF6 + 2HF + 2O2 The high reactivity of F2 creates difficulties with the choice of con- structural materials for working with it. Usually, for these purposes, They contain nickel and copper, which, when oxidized, form dense protective films of fluorides on their surface. The name F is associated with its aggressive action. I mean, it comes from the Greek. "Ftoros" - "destroying". In the series F2, Cl2, Br2, I2, the oxidizing ability weakens due to an increase in changing the size of atoms and reducing electronegativity. In aqueous solutions, the oxidizing and reducing properties of substances are usually characterized using electrode potentials. The table shows the standard electrode potentials (Eo, V) for the half-reactions of the formation of halogens. For comparison, the value of Eo for ki- oxygen is the most common oxidizing agent. Standard electrode potentials for simple substances halogens
Decreased oxidative activity As can be seen from the table, F2 - the oxidizing agent is much stronger, than O2, therefore F2 does not exist in aqueous solutions , it oxidizes water, recovering to F–. Judging by the value of Eo, the oxidizing ability of Cl2
also higher than that of O2. Indeed, during long-term storage of chlorine water, it decomposes with the release of oxygen and with the formation of HCl. But the reaction is slow (the Cl2 molecule is noticeably stronger than the F2 molecule and activation energy for reactions with chlorine is higher), dispro- portioning: Cl2 + H2 O HCl + HOCl In water, it does not reach the end (K = 3.9.10–4), therefore Cl2 exists in aqueous solutions. Br2 and I2 are even more stable in water. Disproportionation is a very characteristic oxidative reduction reaction for halogens. The disproportionation of the poured in an alkaline environment. Disproportionation of Cl2 in alkali leads to the formation of anions Cl– and ClO– . The disproportionation constant is 7.5. 1015 . Cl2 + 2NaOH = NaCl + NaClO + H2O When iodine is disproportionated in alkali, I– and IO3 – are formed. Ana- Br2 disproportionates iodine logically. The change in the product is disproportionate The ionization is due to the fact that the anions GO– and GO2 – in Br and I are unstable. The disproportionation reaction of chlorine is used in industrial sti to obtain a strong and fast-acting hypochlorite oxidizing agent, bleaching lime, bartholite salt. 3Cl2 + 6KOH = 5KCl + KClO3 + 3H2O
Interaction of halogens with metals Halogens interact vigorously with many metals, for example: Mg + Cl2 = MgCl2 Ti + 2I2 TiI4 Na + halides, in which the metal has a low oxidation state (+1, +2), are salt-like compounds with a predominantly ionic bond. How to- lo, ionic halides are solids with a high melting point Metal halides, in which the metal has a high oxidation state niya, are compounds with a predominantly covalent bond. Many of them under normal conditions are gases, liquids or fusible solids. For example, WF6 is a gas, MoF6 is a liquid, TiCl4 is a liquid. Interaction of halogens with non-metals Halogens interact directly with many non-metals: hydrogen, phosphorus, sulfur, etc. For example: H2 + Cl2 = 2HCl 2P + 3Br2 = 2PBr3 S + 3F2 = SF6 The bond in non-metal halides is predominantly covalent. These compounds usually have low melting and boiling points. In the transition from fluorine to iodine, the covalent character of the halides is enhanced. Covalent halides of typical non-metals are acidic compounds; when interacting with water, they hydrolyze to form acids. For example: PBr3 + 3H2O = 3HBr + H3PO3 PI3 + 3H2O = 3HI + H3PO3 PCl5 + 4H2O = 5HCl + H3PO4
The first two reactions are used to obtain bromine and hydrogen iodide noic acid. Interhalides. Halogens, combining with each other, form an inter- leads. In these compounds, the lighter and more electronegative halogen is in the oxidation state (–1), and the heavier one is in the positive state. oxidation foam. Due to the direct interaction of halogens when heated, the following are obtained: ClF, BrF, BrCl, ICl. There are also more complex interhalides: ClF3 , BrF3 , BrF5 , IF5 , IF7 , ICl3 . All interhalides under normal conditions are liquid substances with low boiling points. Interhalides have a high oxidizing activity. For example, such chemically stable substances as SiO2, Al2 O3, MgO, etc. burn in ClF3 vapors. 2Al2O3 + 4ClF3 = 4AlF3 + 3O2 + 2Cl2 Fluoride ClF 3 is an aggressive fluorinating reagent that acts quickly yard F2 . It is used in organic syntheses and to obtain protective films on the surface of nickel equipment for working with fluorine. In water, interhalides are hydrolyzed to form acids. For example, ClF5 + 3H2O = HClO3 + 5HF Halogens in nature. Obtaining simple substances In industry, halogens are obtained from their natural compounds. All processes for obtaining free halogens are based on the oxidation of halo- nid ions. 2D – Г2 + 2e– A significant amount of halogens is found in natural waters in the form of anions: Cl–, F–, Br–, I–. Sea water can contain up to 2.5% NaCl. Bromine and iodine are obtained from oil well water and sea water.
The structure and properties of atoms. The elements of the main subgroup of group VII of the Periodic system of D. I. Mendeleev, united under the general name halogens - fluorine F, chlorine Cl, bromine Br, iodine I, astatine At (rarely found in nature) are typical non-metals. This is understandable, because their atoms contain seven electrons in the outer energy level, and they lack only one electron to complete it. Halogen atoms, when interacting with metals, accept an electron from metal atoms. In this case, an ionic bond occurs and salts are formed. Hence the common name of the subgroup "halogens", i.e. "giving birth to salts." Halogens are very strong oxidizing agents. Fluorine in chemical reactions exhibits only oxidizing properties, and it is characterized only by the oxidation state -1 in compounds. The remaining halogens also exhibit reducing properties when interacting with more electronegative elements - fluorine, oxygen, nitrogen. Their oxidation states can take on the values +1, +3, +5, +7. The reducing properties of halogens increase from chlorine to iodine, which is associated with an increase in the radii of their atoms: chlorine atoms are about one and a half times smaller than iodine. Halogens are simple substances. All halogens exist in the free state as diatomic molecules with a covalent non-polar chemical bond between the atoms. In the solid state, F 2 , Cl 2 , Br 2 , I 2 have molecular crystal lattices, which is confirmed by their physical properties (Table 7). Table 7
As you can see, with an increase in the molecular weight of halogens, their melting and boiling points increase (Fig. 88), the density increases: fluorine and chlorine are gases, bromine is a liquid, iodine is a solid.
Rice. 88. This is due to the fact that with an increase in the size of atoms and molecules of halogens (Fig. 89), the forces of intermolecular interaction between them also increase.
Rice. 89. From F 2 to I 2, the color intensity of the halogens increases. Iodine crystals have a metallic sheen. The chemical activity of halogens, as non-metals, weakens from fluorine to iodine. Each halogen is the strongest oxidizing agent in its period. The oxidizing properties of halogens are clearly manifested when they interact with metals. In this case, as you already know, salts are formed. Thus, fluorine already under normal conditions reacts with most metals, and when heated - with gold, silver, platinum, known for their chemical passivity. Aluminum and zinc ignite in a fluorine atmosphere:
The remaining halogens react with metals mainly when heated. So, in a flask filled with chlorine, crystals of crushed antimony flare up and burn beautifully (Fig. 90), while forming a mixture of two antimony chlorides (III) and (V):
Rice. 90. Heated iron powder also ignites when interacting with chlorine. The experiment can also be carried out with antimony, but only iron filings must first be heated in an iron spoon, and then poured in small portions into a flask with chlorine. Since chlorine is a strong oxidizing agent, iron (III) chloride is formed as a result of the reaction (Fig. 91):
Rice. 91. Hot copper wire burns in bromine vapor:
Iodine oxidizes metals more slowly, but in the presence of water, which is a catalyst, the reaction of iodine with aluminum powder proceeds very rapidly:
The reaction is accompanied by the release of violet vapors of iodine (why?). The decrease in the oxidizing and increase in the reducing properties of halogens from fluorine to iodine can also be judged by their ability to displace each other from salt solutions.
Rice. 92. Free bromine displaces iodine from salts:
For fluorine, this reaction is not typical, since it occurs in solution, and fluorine interacts with water, displacing oxygen from it:
Here, oxygen plays an unusual role as a reducing agent. This is perhaps the only case when oxygen in the combustion reaction is not one of the initial substances, but its product. The weakening of the oxidizing properties of halogens from fluorine to iodine is clearly manifested when they interact with hydrogen. The equation for this reaction can be written in general form: H 2 + G 2 \u003d 2NG (G - conventional chemical designation of halogens). If fluorine interacts with hydrogen under any conditions with an explosion, then a mixture of chlorine with hydrogen reacts with an explosion only when ignited or irradiated with direct sunlight, bromine interacts with hydrogen when heated and without an explosion. These reactions are exothermic. The reaction of the compound of crystalline iodine with hydrogen is weakly endothermic; it proceeds slowly even when heated. As a result of these reactions, hydrogen fluoride HF, hydrogen chloride HCl, hydrogen bromide HBr and hydrogen iodide HI are formed, respectively. Discovery of halogens. Fluorine in free form was first obtained in 1886 by the French chemist A. Moissan, who was awarded the Nobel Prize for this. The element got its name from the Greek fluoros - "destroying". Chlorine was discovered by the Swedish chemist K. Scheele in 1774. The element was named for the color of a simple substance (from the Greek chloros - yellow-green). Bromine was discovered in 1826 by the French chemist A. Balard. The element is named so for the smell of a simple substance (from the Greek. Bromos - fetid). Iodine was obtained in 1811 by the French scientist B. Courtois, and received the name for the color of the vapors of a simple substance (from the Greek iodes - violet). New words and concepts
Tasks for independent work
Read also: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||