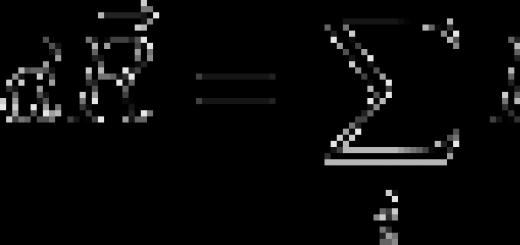The nuclei of atoms are strongly bound systems of a large number of nucleons.
For the complete splitting of the nucleus into its constituent parts and their removal to long distances each other, it is necessary to expend a certain amount of work A.
The binding energy is the energy equal to the work that must be done to split the nucleus into free nucleons.
E bonds = - A
According to the law of conservation, the binding energy is simultaneously equal to the energy that is released during the formation of a nucleus from individual free nucleons.
Specific binding energy
This is the binding energy per nucleon.
Except for the lightest nuclei, the specific binding energy is approximately constant and equal to 8 MeV/nucleon. Elements with mass numbers from 50 to 60 have the maximum specific binding energy (8.6 MeV/nucleon). The nuclei of these elements are the most stable.
As nuclei are overloaded with neutrons, the specific binding energy decreases.
For the elements at the end of the periodic table, it is equal to 7.6 MeV/nucleon (for example, for uranium).
Release of energy as a result of nuclear fission or fusion
In order to split the nucleus, it is necessary to expend a certain energy to overcome nuclear forces.
In order to synthesize a nucleus from individual particles, it is necessary to overcome the Coulomb repulsive forces (for this, energy must be expended to accelerate these particles to high speeds).
That is, in order to carry out the splitting of the nucleus or the fusion of the nucleus, some energy must be expended.
During nuclear fusion at short distances, nuclear forces begin to act on nucleons, which induce them to move with acceleration.
Accelerated nucleons emit gamma quanta, which have an energy equal to the binding energy.
At the output of the nuclear fission reaction or fusion, energy is released.
It makes sense to carry out nuclear fission or nuclear synthesis, if the resulting, i.e. the energy released as a result of splitting or fusion will be greater than the energy expended
According to the graph, the gain in energy can be obtained either by fission (splitting) of heavy nuclei, or by fusion of light nuclei, which is done in practice.
mass defect
Measurements of the masses of nuclei show that the mass of the nucleus (Mn) is always less than the sum of the rest masses of the free neutrons and protons composing it.
During nuclear fission: the mass of the nucleus is always less than the sum of the rest masses of the formed free particles.
In the synthesis of the nucleus: the mass of the formed nucleus is always less than the sum of the rest masses of the free particles that formed it.
The mass defect is a measure of the binding energy atomic nucleus.
The mass defect is equal to the difference between the total mass of all nucleons of the nucleus in the free state and the mass of the nucleus:
where Mm is the mass of the nucleus (from the reference book)
Z is the number of protons in the nucleus
mp is the rest mass of a free proton (from the handbook)
N is the number of neutrons in the nucleus
mn is the rest mass of a free neutron (from the handbook)
The decrease in mass during the formation of a nucleus means that the energy of the system of nucleons decreases.
Nucleus Binding Energy Calculation
The nuclear binding energy is numerically equal to the work that must be expended to split the nucleus into individual nucleons, or the energy released during the synthesis of nuclei from nucleons.
The measure of the nuclear binding energy is the mass defect.
The formula for calculating the binding energy of a nucleus is Einstein's formula:
if there is some system of particles that has mass, then a change in the energy of this system leads to a change in its mass.
Here, the binding energy of the nucleus is expressed as the product of the mass defect and the square of the speed of light.
In nuclear physics, the mass of particles is expressed in atomic mass units (a.m.u.)
in nuclear physics, it is customary to express energy in electronvolts (eV):
Let's calculate the correspondence of 1 a.m.u. electronvolts:
Now the calculation formula for the binding energy (in electronvolts) will look like this:
EXAMPLE OF CALCULATION OF THE BINDING ENERGY OF THE NUCLEI OF A HELIUM ATOM (He)
>nuclear forces
In order for atomic nuclei to be stable, protons and neutrons must be held inside the nuclei by huge forces, many times greater than the Coulomb repulsive forces of protons. The forces that hold nucleons in the nucleus are called nuclear . They are a manifestation of the most intense of all types of interaction known in physics - the so-called strong interaction. The nuclear forces are about 100 times greater than the electrostatic forces and are tens of orders of magnitude greater than the forces of the gravitational interaction of nucleons.
Nuclear forces have the following properties:
have attractive forces
is a force short-range(appear at small distances between nucleons);
Nuclear forces do not depend on the presence or absence of an electric charge on particles.
Mass Defect and Binding Energy of the Nucleus of an Atom
The most important role in nuclear physics is played by the concept nuclear binding energy .
The binding energy of the nucleus is equal to the minimum energy that must be expended for the complete splitting of the nucleus into individual particles. It follows from the law of conservation of energy that the binding energy is equal to the energy that is released during the formation of a nucleus from individual particles.
The binding energy of any nucleus can be determined by accurately measuring its mass. At present, physicists have learned to measure the masses of particles - electrons, protons, neutrons, nuclei, etc. - with very high accuracy. These measurements show that the mass of any nucleus M i is always less than the sum of the masses of its constituent protons and neutrons:
The mass difference is called mass defect. Based on the mass defect using the Einstein formula E = mc 2 it is possible to determine the energy released during the formation of a given nucleus, i.e., the binding energy of the nucleus E St:
This energy is released during the formation of the nucleus in the form of radiation of γ-quanta.
B21 1), B22 1), B23 1), B24 1), B25 2)
A magnetic field
If two parallel conductors are connected to a current source so that electricity, then, depending on the direction of the current in them, the conductors either repel or attract.
The explanation of this phenomenon is possible from the standpoint of the appearance around the conductors of a special type of matter - a magnetic field.
The forces with which current-carrying conductors interact are called magnetic.
A magnetic field- This special kind matter, a specific feature of which is the action on a moving electric charge, conductors with current, bodies with a magnetic moment, with a force depending on the charge velocity vector, the direction of the current strength in the conductor and on the direction of the magnetic moment of the body.
The history of magnetism goes back to ancient times, to the ancient civilizations of Asia Minor. It was on the territory of Asia Minor, in Magnesia, that a rock was found, samples of which were attracted to each other. According to the name of the area, such samples began to be called "magnets". Any magnet in the form of a rod or a horseshoe has two ends, which are called poles; it is in this place that its magnetic properties are most pronounced. If you hang a magnet on a string, one pole will always point north. The compass is based on this principle. The north-facing pole of a free-hanging magnet is called the magnet's north pole (N). The opposite pole is called south pole(S).
Magnetic poles interact with each other: like poles repel, and opposite poles attract. Similar to the concept electric field, surrounding an electric charge, introduce the concept of a magnetic field around a magnet.
In 1820, Oersted (1777-1851) discovered that a magnetic needle located next to electrical conductor, deviates when current flows through the conductor, i.e., a magnetic field is created around the current-carrying conductor. If we take a loop with current, then the external magnetic field interacts with magnetic field frame and has an orienting effect on it, that is, there is a position of the frame in which the external magnetic field has a maximum rotating effect on it, and there is a position when the torque of the forces is zero.
The magnetic field at any point can be characterized by the vector B, which is called magnetic induction vector or magnetic induction at the point.
Magnetic induction B is a vector physical quantity, which is the force characteristic of the magnetic field at the point. It is equal to the ratio of the maximum mechanical moment of forces acting on a loop with current placed in a uniform field to the product of the current strength in the loop and its area:
![]()
The direction of the magnetic induction vector B is taken to be the direction of the positive normal to the frame, which is related to the current in the frame by the rule of the right screw, with a mechanical moment equal to zero.
In the same way as the lines of electric field strength are depicted, the lines of magnetic field induction are depicted. The line of induction of the magnetic field is an imaginary line, the tangent to which coincides with the direction B at the point.
The directions of the magnetic field at a given point can also be defined as the direction that indicates
the north pole of the compass needle placed at that point. It is believed that the lines of induction of the magnetic field are directed from north pole to the south.
The direction of the lines of magnetic induction of the magnetic field created by an electric current that flows through a straight conductor is determined by the rule of a gimlet or a right screw. The direction of rotation of the screw head is taken as the direction of the lines of magnetic induction, which would ensure its translational movement in the direction of the electric current (Fig. 59).

![]()
where n 01 = 4 Pi 10 -7 V s / (A m). - magnetic constant, R - distance, I - current strength in the conductor.
Unlike electrostatic field lines, which start at a positive charge and end at a negative one, magnetic field lines are always closed. Magnetic charge is similar electric charge not found.
One tesla (1 T) is taken as a unit of induction - the induction of such a uniform magnetic field in which a maximum rotating mechanical moment forces equal to 1 N m.
![]()
The induction of a magnetic field can also be determined by the force acting on a current-carrying conductor in a magnetic field.
A conductor with current placed in a magnetic field is subjected to the Ampère force, the value of which is determined by the following expression:
where I is the current strength in the conductor, l- the length of the conductor, B is the modulus of the magnetic induction vector, and is the angle between the vector and the direction of the current.
The direction of the Ampere force can be determined by the rule of the left hand: the palm of the left hand is positioned so that the lines of magnetic induction enter the palm, four fingers are placed in the direction of the current in the conductor, then the bent thumb shows the direction of the Ampere force.
Considering that I = q 0 nSv and substituting this expression into (3.21), we obtain F = q 0 nSh/B sin a. The number of particles (N) in a given volume of the conductor is N = nSl, then F = q 0 NvB sin a.
Let us determine the force acting from the side of the magnetic field on a separate charged particle moving in a magnetic field:
![]()
This force is called the Lorentz force (1853-1928). The direction of the Lorentz force can be determined by the rule of the left hand: the palm of the left hand is positioned so that the lines of magnetic induction enter the palm, four fingers show the direction of movement of the positive charge, the thumb bent shows the direction of the Lorentz force.
The force of interaction between two parallel conductors, through which currents I 1 and I 2 flow, is equal to:
where l- the part of a conductor that is in a magnetic field. If the currents are in the same direction, then the conductors are attracted (Fig. 60), if the opposite direction, they are repelled. The forces acting on each conductor are equal in magnitude, opposite in direction. Formula (3.22) is the main one for determining the unit of current strength 1 ampere (1 A).
The magnetic properties of a substance are characterized by a scalar physical quantity - magnetic permeability, which shows how many times the induction B of a magnetic field in a substance that completely fills the field differs in absolute value from the induction B 0 of a magnetic field in vacuum:

According to their magnetic properties, all substances are divided into diamagnetic, paramagnetic and ferromagnetic.
Consider nature magnetic properties substances.
Electrons in the shell of atoms of matter move in different orbits. For simplicity, we consider these orbits to be circular, and each electron revolving around the atomic nucleus can be considered as a circular electric current. Each electron, like a circular current, creates a magnetic field, which we will call orbital. In addition, an electron in an atom has its own magnetic field, called the spin field.
If, when introduced into an external magnetic field with induction B 0, induction B is created inside the substance< В 0 , то такие вещества называются диамагнитными (n< 1).
AT diamagnetic In materials in the absence of an external magnetic field, the magnetic fields of electrons are compensated, and when they are introduced into a magnetic field, the induction of the magnetic field of an atom becomes directed against the external field. The diamagnet is pushed out of the external magnetic field.
At paramagnetic materials, the magnetic induction of electrons in atoms is not fully compensated, and the atom as a whole turns out to be like a small permanent magnet. Usually in matter all these small magnets are oriented arbitrarily, and the total magnetic induction of all their fields is equal to zero. If you place a paramagnet in an external magnetic field, then all small magnets - atoms will turn in the external magnetic field like compass needles and the magnetic field in the substance increases ( n >= 1).
ferromagnetic are materials that are n"1. So-called domains, macroscopic regions of spontaneous magnetization, are created in ferromagnetic materials.
In different domains, the induction of magnetic fields has different directions (Fig. 61) and in a large crystal


mutually compensate each other. When a ferromagnetic sample is introduced into an external magnetic field, the boundaries of individual domains are shifted so that the volume of domains oriented along the external field increases.
With an increase in the induction of the external field B 0, the magnetic induction of the magnetized substance increases. For some values of B 0, the induction stops its sharp growth. This phenomenon is called magnetic saturation.
Feature ferromagnetic materials - the phenomenon of hysteresis, which consists in the ambiguous dependence of the induction in the material on the induction of the external magnetic field when it changes.
The magnetic hysteresis loop is a closed curve (cdc`d`c), expressing the dependence of the induction in the material on the amplitude of the induction of the external field with a periodic rather slow change in the latter (Fig. 62).
The hysteresis loop is characterized by the following values B s , B r , B c . B s - the maximum value of the induction of the material at B 0s ; B r - residual induction, equal to the value of the induction in the material when the induction of the external magnetic field decreases from B 0s to zero; -B c and B c - coercive force - a value equal to the induction of the external magnetic field necessary to change the induction in the material from residual to zero.
For each ferromagnet, there is such a temperature (Curie point (J. Curie, 1859-1906), above which the ferromagnet loses its ferromagnetic properties.
There are two ways to bring a magnetized ferromagnet into a demagnetized state: a) heat above the Curie point and cool; b) magnetize the material with an alternating magnetic field with a slowly decreasing amplitude.
Ferromagnets with low residual induction and coercive force are called soft magnetic. They find application in devices where a ferromagnet has to be frequently remagnetized (cores of transformers, generators, etc.).
Magnetically hard ferromagnets, which have a large coercive force, are used for the manufacture of permanent magnets.
B21 2) Photoelectric effect. Photons
photoelectric effect was discovered in 1887 by the German physicist G. Hertz and experimentally studied by A. G. Stoletov in 1888–1890. The most complete study of the phenomenon of the photoelectric effect was carried out by F. Lenard in 1900. By this time, the electron had already been discovered (1897, J. Thomson), and it became clear that the photoelectric effect (or, more precisely, the external photoelectric effect) consists in pulling electrons out of matter under the influence of light falling on it.
The layout of the experimental setup for studying the photoelectric effect is shown in fig. 5.2.1.
The experiments used a glass vacuum vessel with two metal electrodes, the surface of which was thoroughly cleaned. A voltage was applied to the electrodes U, the polarity of which could be changed using a double key. One of the electrodes (cathode K) was illuminated through a quartz window with monochromatic light of a certain wavelength λ. At a constant luminous flux, the dependence of the photocurrent strength was taken I from the applied voltage. On fig. 5.2.2 shows typical curves of such a dependence obtained at two intensity values luminous flux incident on the cathode.
The curves show that at sufficiently high positive voltages at the anode A, the photocurrent reaches saturation, since all the electrons ejected by light from the cathode reach the anode. Careful measurements have shown that the saturation current I n is directly proportional to the intensity of the incident light. When the voltage across the anode is negative, the electric field between the cathode and anode slows down the electrons. The anode can only reach those electrons whose kinetic energy exceeds | EU|. If the anode voltage is less than - U h, the photocurrent stops. measuring U h, it is possible to determine the maximum kinetic energy of photoelectrons:
Numerous experimenters have established the following basic laws of the photoelectric effect:
- The maximum kinetic energy of photoelectrons increases linearly with increasing light frequency ν and does not depend on its intensity.
- For every substance there is a so-called red border photo effect , i.e., the lowest frequency ν min at which an external photoelectric effect is still possible.
- The number of photoelectrons pulled out by light from the cathode in 1 s is directly proportional to the light intensity.
- The photoelectric effect is practically inertialess, the photocurrent appears instantly after the start of cathode illumination, provided that the light frequency ν > ν min .
All these laws of the photoelectric effect fundamentally contradicted the ideas of classical physics about the interaction of light with matter. According to wave concepts, when interacting with an electromagnetic light wave, an electron would have to gradually accumulate energy, and it would take a considerable time, depending on the intensity of light, for the electron to accumulate enough energy to fly out of the cathode. Calculations show that this time should have been calculated in minutes or hours. However, experience shows that photoelectrons appear immediately after the start of illumination of the cathode. In this model, it was also impossible to understand the existence of the red boundary of the photoelectric effect. The wave theory of light could not explain the independence of the energy of photoelectrons from the intensity of the light flux and the proportionality of the maximum kinetic energy to the frequency of light.
Thus, the electromagnetic theory of light proved unable to explain these regularities.
The way out was found by A. Einstein in 1905. Theoretical explanation of the observed laws of the photoelectric effect was given by Einstein on the basis of M. Planck's hypothesis that light is emitted and absorbed in certain portions, and the energy of each such portion is determined by the formula E = h v, where h is Planck's constant. Einstein took the next step in the development of quantum concepts. He came to the conclusion that light has a discontinuous (discrete) structure. electromagnetic wave consists of separate portions - quanta, subsequently named photons. When interacting with matter, a photon transfers all of its energy hν to one electron. Part of this energy can be dissipated by an electron in collisions with atoms of matter. In addition, part of the electron energy is spent on overcoming the potential barrier at the metal–vacuum interface. To do this, the electron must do the work function A depending on the properties of the cathode material. The maximum kinetic energy that a photoelectron emitted from the cathode can have is determined by the energy conservation law:
|
This formula is called Einstein's equation for the photoelectric effect .
Using the Einstein equation, one can explain all the regularities of the external photoelectric effect. From the Einstein equation, the linear dependence of the maximum kinetic energy on frequency and independence on light intensity, the existence of a red border, and the inertia of the photoelectric effect follow. Total number photoelectrons leaving the cathode surface in 1 s should be proportional to the number of photons incident on the surface in the same time. It follows from this that the saturation current must be directly proportional to the intensity of the light flux.
As follows from the Einstein equation, the slope of the straight line expressing the dependence of the blocking potential U h from the frequency ν (Fig. 5.2.3), is equal to the ratio Planck's constant h to the charge of an electron e:
where c is the speed of light, λcr is the wavelength corresponding to the red border of the photoelectric effect. For most metals, the work function A is a few electron volts (1 eV = 1.602 10 -19 J). AT quantum physics The electron volt is often used as an energy unit of measure. The value of Planck's constant, expressed in electron volts per second, is
Among metals, alkaline elements have the lowest work function. For example, sodium A= 1.9 eV, which corresponds to the red border of the photoelectric effect λcr ≈ 680 nm. Therefore, connections alkali metals used to create cathodes in photocells designed to detect visible light.
So, the laws of the photoelectric effect indicate that light, when emitted and absorbed, behaves like a stream of particles called photons or light quanta .
The photon energy is
it follows that the photon has momentum
| |
Thus, the doctrine of light, having completed a revolution lasting two centuries, again returned to the ideas of light particles - corpuscles.
But it was not a mechanical return to corpuscular theory Newton. At the beginning of the 20th century, it became clear that light has a dual nature. When light propagates, its wave properties appear (interference, diffraction, polarization), and when interacting with matter, corpuscular (photoelectric effect). This dual nature of light is called wave-particle duality . Later, the dual nature was discovered in electrons and other elementary particles. Classical physics cannot give a visual model of the combination of wave and corpuscular properties at micro-objects. The motion of micro-objects is controlled not by the laws of classical Newtonian mechanics, but by the laws quantum mechanics. The black body radiation theory developed by M. Planck and Einstein's quantum theory of the photoelectric effect underlie this modern science.
B23 2) special theory Relativity, like any other physical theory, can be formulated on the basis of the basic concepts and postulates (axioms) plus the rules of correspondence to its physical objects.
Basic concepts[edit | edit wiki text]
A reference system is a certain material body chosen as the beginning of this system, a method for determining the position of objects relative to the origin of the reference system, and a method for measuring time. A distinction is usually made between reference systems and coordinate systems. Adding a procedure for measuring time to a coordinate system "turns" it into a reference system.
An inertial reference system (ISR) is such a system, relative to which an object, not subject to external influences, moves uniformly and rectilinearly. It is postulated that IFRs exist, and any frame of reference moving uniformly and rectilinearly relative to a given inertial frame is also an IFR.
An event is any physical process that can be localized in space and has a very short duration. In other words, the event is fully characterized by coordinates (x, y, z) and time t. Examples of events are: flash of light, position material point in this moment time, etc.
Two are usually considered inertial systems S and S". The time and coordinates of some event, measured with respect to the S system, are denoted as (t, x, y, z), and the coordinates and time of the same event, measured with respect to the S" system, as (t", x", y", z"). It is convenient to assume that the coordinate axes of the systems are parallel to each other, and the system S" moves along the x-axis of the system S with a velocity v. One of the tasks of SRT is to find relationships connecting (t", x", y", z") and (t , x, y, z), which are called Lorentz transformations.
Time synchronization[edit | edit wiki text]
SRT postulates the possibility of determining a single time within a given inertial frame of reference. To do this, a synchronization procedure is introduced for two clocks located at different points of the ISO. Let a signal (not necessarily light) be sent from the first clock at the time (\displaystyle t_(1)) to the second clock at a constant speed (\displaystyle u) . Immediately upon reaching the second clock (according to their reading at time (\displaystyle T)) the signal is sent back at the same constant rate (\displaystyle u) and reaches the first clock at time (\displaystyle t_(2)) . Clocks are considered synchronized if (\displaystyle T=(t_(1)+t_(2))/2) holds.
It is assumed that such a procedure in a given inertial reference frame can be carried out for any clocks that are stationary relative to each other, so the transitivity property is true: if the clocks A synchronized with clock B, and the clock B synchronized with clock C, then the clock A and C will also be synchronized.
Unlike classical mechanics, a single time can only be introduced within a given frame of reference. SRT does not assume that time is common to different systems. This is the main difference between the SRT axiomatics and classical mechanics, which postulates the existence of a single (absolute) time for all frames of reference.
Coordination of units of measurement[edit | edit wiki text]
In order for measurements made in different ISOs to be compared with each other, it is necessary to coordinate the units of measurement between reference systems. Thus, units of length can be agreed upon by comparing length standards in a direction perpendicular to relative motion inertial reference systems. For example, it could be shortest distance between the trajectories of two particles moving parallel to the axes x and x "and having different but constant coordinates (y, z) and (y", z"). To coordinate the units of time, you can use identically arranged clocks, for example, atomic clocks.
SRT postulates[edit | edit wiki text]
First of all, in SRT, as in classical mechanics, it is assumed that space and time are homogeneous, and space is also isotropic. To be more precise (modern approach), inertial frames of reference are actually defined as such frames of reference in which space is homogeneous and isotropic, and time is homogeneous. In fact, the existence of such reference systems is postulated.
Postulate 1 (Einstein's principle of relativity). The laws of nature are the same in all coordinate systems moving in a straight line and uniformly relative to each other. It means that the form the dependence of physical laws on space-time coordinates should be the same in all IFRs, that is, the laws are invariant with respect to transitions between IFRs. The principle of relativity establishes the equality of all ISOs.
Taking into account Newton's second law (or the Euler-Lagrange equations in Lagrangian mechanics), it can be argued that if the speed of a certain body in a given IFR is constant (acceleration is zero), then it must be constant in all other IFRs. Sometimes this is taken as the definition of ISO.
Formally, Einstein's principle of relativity extended the classical principle of relativity (Galileo) from mechanical to all physical phenomena. However, if we take into account that in the time of Galileo physics consisted of mechanics proper, then the classical principle can also be considered as extending to all physical phenomena. It should also apply to electromagnetic phenomena, described by Maxwell's equations. However, according to the latter (and this can be considered empirically established, since the equations are derived from empirically identified regularities), the speed of light propagation is a certain quantity that does not depend on the speed of the source (at least in one frame of reference). The principle of relativity in this case says that it should not depend on the speed of the source in all IFRs due to their equality. This means that it must be constant in all ISOs. This is the essence of the second postulate:
Postulate 2 (principle of constancy of the speed of light). The speed of light in vacuum is the same in all coordinate systems moving rectilinearly and uniformly relative to each other.
The principle of the constancy of the speed of light contradicts classical mechanics, and specifically, the law of addition of velocities. When deriving the latter, only the principle of Galileo's relativity and the implicit assumption of the same time in all IFRs are used. Thus, it follows from the validity of the second postulate that time must be relative- not the same in different ISOs. It necessarily follows that "distances" must also be relative. In fact, if light travels a distance between two points in a certain time, and in another system - in another time and, moreover, with the same speed, then it immediately follows that the distance in this system must also differ.
It should be noted that light signals, generally speaking, are not required when substantiating SRT. Although the non-invariance of Maxwell's equations with respect to Galilean transformations led to the construction of SRT, the latter has a more general character and is applicable to all types of interactions and physical processes. The fundamental constant (\displaystyle c) that occurs in Lorentz transformations makes sense marginal movement speed material bodies. Numerically, it coincides with the speed of light, but this fact, according to modern quantum field theory (whose equations are initially constructed as relativistically invariant ones), is associated with masslessness electromagnetic field(photon). Even if the photon had a non-zero mass, the Lorentz transformations would not change from this. Therefore, it makes sense to distinguish between the fundamental speed (\displaystyle c) and the speed of light (\displaystyle c_(em)) . The first constant reflects general properties space and time, while the second is associated with the properties of a particular interaction.
The postulate of causality is also used: any event can only affect events that occur after it and cannot affect events that occur before it. From the postulate of causality and the independence of the speed of light from the choice of reference frame, it follows that the speed of any signal cannot exceed the speed of light
B24 2) Basic concepts of nuclear physics. Radioactivity. Types of radioactive decay.
Nuclear physics is a branch of physics that studies the structure and properties of atomic nuclei. Nuclear physics is also concerned with the study of the interconversions of atomic nuclei, which take place both as a result of radioactive decays and as a result of various nuclear reactions. Its main task is connected with the elucidation of the nature of the nuclear forces acting between nucleons and the peculiarities of the motion of nucleons in nuclei. Protons and neutrons- these are the main elementary particles that make up the nucleus of an atom. Nucleon is a particle that has two different charge states: a proton and a neutron. Core charge- the number of protons in the nucleus, the same as the atomic number of the element in periodic system Mendeleev. isotopes- nuclei having the same charge, if the mass number of nucleons is different.
isobars- these are nuclei with the same number of nucleons, with different charges.
Nuclide is a specific kernel with values. Specific binding energy is the binding energy per nucleon of the nucleus. It is determined experimentally. Ground State of the Kernel- this is the state of the nucleus, which has the lowest possible energy, equal to the binding energy. Excited state of the nucleus- this is the state of the nucleus, which has energy, a large binding energy. Corpuscular-wave dualism. photoelectric effect Light has a dual corpuscular-wave nature, i.e. corpuscular-wave dualism: firstly: it has wave properties; secondly: it acts as a stream of particles - photons. Electromagnetic radiation is not only emitted by quanta, but propagates and is absorbed in the form of particles (corpuscles) of the electromagnetic field - photons. Photons are actually existing particles of the electromagnetic field. Quantization is a method of selecting electron orbits corresponding to the stationary states of an atom.
RADIOACTIVITY
Radioactivity - called the ability of the atomic nucleus to spontaneously decay with the emission of particles. Spontaneous decay of isotopes of nuclei under conditions natural environment called natural radioactivity - it is the radioactivity that can be observed in naturally occurring unstable isotopes. And in the conditions of laboratories as a result of human activity – artificial radioactivity - is the radioactivity of isotopes acquired as a result of nuclear reactions. Radioactivity is accompanied
the transformation of one chemical element in another and is always accompanied by the release of energy. Quantitative estimates are established for each radioactive element. So, the probability of decay of one atom in one second is characterized by the decay constant of this element, and the time for which half of the radioactive sample decays is called the half-life. The number of radioactive decays in the sample in one second is called the activity of the radioactive drug. The unit of activity in the SI system is Becquerel (Bq): 1 Bq = 1 decay / 1 s.
radioactive decay is a process that is static, in which the nuclei of a radioactive element decay independently of each other. TYPES OF RADIOACTIVE DECAY
The main types of radioactive decay are:
Alpha - decay
Alpha particles are emitted only by heavy nuclei, i.e. containing big number protons and neutrons. The strength of heavy nuclei is low. In order to leave the nucleus, the nucleon must overcome the nuclear forces, and for this it must have sufficient energy. When combining two protons and two neutrons into an alpha particle, the nuclear forces in such a combination are the strongest, and the bonds with other nucleons are weaker, so the alpha particle is able to "escape" from the nucleus. The emitted alpha particle carries away a positive charge of 2 units and a mass of 4 units. As a result of alpha decay, a radioactive element turns into another element, serial number which is 2 units, and the mass number is 4 units, less. That nucleus, which decays, is called the parent, and the formed child. The daughter nucleus is usually also radioactive and decays after a while. The process of radioactive decay proceeds until a stable nucleus, most often a lead or bismuth nucleus, appears.
Studies show that atomic nuclei are stable formations. This means that there is a certain connection between nucleons in the nucleus. The study of this connection can be carried out without drawing on information about the nature and properties of nuclear forces, but based on the law of conservation of energy.
Let's introduce definitions.
The binding energy of the nucleon in the nucleus called a physical quantity equal to work, which must be done to remove a given nucleon from the nucleus without imparting kinetic energy to it.
Complete core binding energy is determined by the work that must be done to split the nucleus into its constituent nucleons without imparting kinetic energy to them.
It follows from the law of conservation of energy that during the formation of a nucleus, an energy equal to the binding energy of the nucleus must be released from its constituent nucleons. Obviously, the binding energy of the nucleus is equal to the difference between the total energy of the free nucleons that make up the given nucleus and their energy in the nucleus.
From the theory of relativity it is known that there is a relationship between energy and mass:
E \u003d mc 2. (250)
If through ΔE sv denote the energy released during the formation of the nucleus, then this release of energy, according to formula (250), should be associated with a decrease in the total mass of the nucleus during its formation from constituent particles:
Δm = ΔE sv / since 2 (251)
If denoted by m p , m n , m I respectively the masses of the proton, neutron and nucleus, then ∆m can be determined by the formula:
Dm = [Zm p + (A-Z)m n]- m I . (252)
The mass of nuclei can be determined very accurately using mass spectrometers - measuring instruments, separating beams of charged particles (usually ions) with different specific charges q/m. Mass spectrometric measurements showed that, indeed, the mass of the nucleus is less than the sum of the masses of its constituent nucleons.
The difference between the sum of the masses of the nucleons that make up the nucleus and the mass of the nucleus is called nuclear mass defect(formula (252)).
According to formula (251), the binding energy of nucleons in a nucleus is determined by the expression:
ΔЕ CB = [Zm p+ (A-Z)m n - m I ]with 2 . (253)
The tables usually do not give the masses of the nuclei m I, and the masses of atoms m a. Therefore, for the binding energy, the formula is used:
ΔE SW =[Zm H+ (A-Z)m n - m a ]with 2 (254)
where m H- mass of a hydrogen atom 1 H 1 . As m H more m p, by the value of the electron mass me, then the first term in square brackets includes the mass Z of electrons. But since the mass of an atom m a different from the mass of the nucleus m I just on the mass Z of electrons, then calculations using formulas (253) and (254) lead to the same results.
Often, instead of the binding energy of the nucleus, one considers specific bond energydЕ CB is the binding energy per one nucleon of the nucleus. It characterizes the stability (strength) of atomic nuclei, i.e., the more dЕ CB, the more stable the core . The specific binding energy depends on the mass number BUT element. For light nuclei (A £ 12), the specific binding energy rises steeply to 6 ¸ 7 MeV, undergoing a series of jumps (see Figure 93). For example, for dЕ CB= 1.1 MeV, for -7.1 MeV, for -5.3 MeV. With a further increase in the mass number dE, the SW increases more slowly to a maximum value of 8.7 MeV for elements with BUT=50¸60, and then gradually decreases for heavy elements. For example, for it is 7.6 MeV. Note for comparison that the binding energy of valence electrons in atoms is about 10 eV (10 6 times less).
On the curve of dependence of the specific binding energy on the mass number for stable nuclei (Figure 93), the following patterns can be noted:
a) If we discard the lightest nuclei, then in a rough, so to speak zero approximation, the specific binding energy is constant and equal to approximately 8 MeV per
nucleon. The approximate independence of the specific binding energy from the number of nucleons indicates the saturation property of nuclear forces. This property is that each nucleon can only interact with a few neighboring nucleons.
b) The specific binding energy is not strictly constant, but has a maximum (~8.7 MeV/nucleon) at BUT= 56, i.e. in the area of iron nuclei, and falls to both edges. The maximum of the curve corresponds to the most stable nuclei. It is energetically advantageous for the lightest nuclei to merge with each other with the release of thermonuclear energy. For the heaviest nuclei, on the contrary, the process of fission into fragments is beneficial, which proceeds with the release of energy, called atomic energy.
The most stable are the so-called magic nuclei, in which the number of protons or the number of neutrons is equal to one of the magic numbers: 2, 8, 20, 28, 50, 82, 126. Especially stable are doubly magic nuclei, in which both the number of protons and the number of neutrons. There are only five of these cores: , , , , .
The nucleons inside the nucleus are held together by nuclear forces. They are held by a certain energy. It is quite difficult to measure this energy directly, but it can be done indirectly. It is logical to assume that the energy required to break the bond of nucleons in the nucleus will be equal to or greater than the energy that holds the nucleons together.
Binding Energy and Nuclear Energy
This applied energy is already easier to measure. It is clear that this value will very accurately reflect the value of the energy that keeps the nucleons inside the nucleus. Therefore, the minimum energy required to split the nucleus into individual nucleons is called nuclear binding energy.
Relationship between mass and energy
We know that any energy is directly proportional to the mass of the body. Therefore, it is natural that the binding energy of the nucleus will also depend on the mass of the particles that make up this nucleus. This relationship was established by Albert Einstein in 1905. It is called the law of the relationship between mass and energy. In accordance with this law, the internal energy of a system of particles or the rest energy is directly proportional to the mass of the particles that make up this system:
where E is energy, m is mass,
c is the speed of light in vacuum.
Mass defect effect
Now suppose that we have broken the nucleus of an atom into its constituent nucleons, or that we have taken a certain number of nucleons from the nucleus. We expended some energy on overcoming nuclear forces, because we were doing work. In the case of the reverse process - the fusion of the nucleus, or the addition of nucleons to an already existing nucleus, the energy, according to the law of conservation, on the contrary, will be released. When the rest energy of a system of particles changes due to any processes, their mass changes accordingly. Formulas in this case will be as follows:
∆m=(∆E_0)/c^2 or ∆E_0=∆mc^2,
where ∆E_0 is the change in the rest energy of the system of particles,
∆m is the change in the particle mass.
For example, in the case of the fusion of nucleons and the formation of a nucleus, we release energy and reduce the total mass of nucleons. Mass and energy are carried away by the emitted photons. This is the mass defect effect.. The mass of a nucleus is always less than the sum of the masses of the nucleons that make up this nucleus. Numerically, the mass defect is expressed as follows:
∆m=(Zm_p+Nm_n)-M_i,
where M_m is the mass of the nucleus,
Z is the number of protons in the nucleus,
N is the number of neutrons in the nucleus,
m_p is the free proton mass,
m_n is the mass of a free neutron.
The value ∆m in the above two formulas is the value by which the total mass of the particles of the nucleus changes when its energy changes due to rupture or fusion. In the case of synthesis, this quantity will be the mass defect.
Studies show that atomic nuclei are stable formations. This means that there is a certain connection between nucleons in the nucleus. The study of this connection can be carried out without drawing on information about the nature and properties of nuclear forces, but based on the law of conservation of energy. Let's introduce some definitions.
The binding energy of the nucleon in the nucleus called a physical quantity equal to the work that must be done to remove a given nucleon from the nucleus without imparting kinetic energy to it.
Complete core binding energy is determined by the work that must be done to split the nucleus into its constituent nucleons without imparting kinetic energy to them.
It follows from the law of conservation of energy that during the formation of a nucleus, an energy equal to the binding energy of the nucleus must be released from its constituent nucleons. Obviously, the binding energy of the nucleus is equal to the difference between the total energy of the free nucleons that make up the given nucleus and their energy in the nucleus. From the theory of relativity it is known that there is a relationship between energy and mass:
E \u003d mc 2. (250)
If through ΔE sv denote the energy released during the formation of the nucleus, then this release of energy, according to formula (250), should be associated with a decrease in the total mass of the nucleus during its formation from composite particles:
Δm = ΔE sv / since 2 (251)
If denoted by m p , m n , m I the masses of the proton, neutron and nucleus, respectively, then ∆m can be determined by the formula:
Dm = [Zm p + (A-Z)m n]- m I . (252)
The mass of nuclei can be very accurately determined using mass spectrometers - measuring instruments that separate beams of charged particles (usually ions) with different specific charges using electric and magnetic fields q/m. Mass spectrometric measurements showed that, indeed, the mass of the nucleus is less than the sum of the masses of its constituent nucleons.
The difference between the sum of the masses of the nucleons that make up the nucleus and the mass of the nucleus is called nuclear mass defect(formula (252)).
According to formula (251), the binding energy of nucleons in a nucleus is determined by the expression:
ΔЕ CB = [Zm p+ (A-Z)m n - m I ]with 2 . (253)
The tables usually do not give the masses of the nuclei m I, and the masses of atoms m a. Therefore, for the binding energy, the formula is used
ΔE SW =[Zm H+ (A-Z)m n - m a ]with 2 (254)
where m H- mass of a hydrogen atom 1 H 1 . As m H more m p, by the value of the electron mass me, then the first term in square brackets includes the mass Z of electrons. But since the mass of an atom m a different from the mass of the nucleus m I just on the mass Z of electrons, then calculations using formulas (253) and (254) lead to the same results.
Often, instead of the binding energy of the nucleus, one considers specific bond energydЕ CB is the binding energy per nucleon of the nucleus. It characterizes the stability (strength) of atomic nuclei, i.e., the more dЕ CB, the more stable the core . The specific binding energy depends on the mass number BUT element. For light nuclei (A £ 12), the specific binding energy rises steeply to 6 ¸ 7 MeV, undergoing a series of jumps (see Figure 93). For example, for dЕ CB=1.1 MeV, for -7.1 MeV, for -5.3 MeV. With a further increase in the mass number dE, the SW increases more slowly to a maximum value of 8.7 MeV for elements with BUT=50¸60, and then gradually decreases for heavy elements. For example, for it is 7.6 MeV. Note for comparison that the binding energy of valence electrons in atoms is about 10 eV (10 6 times less). On the curve of dependence of the specific binding energy on the mass number for stable nuclei (Figure 93), the following patterns can be noted:
A) If we discard the lightest nuclei, then in a rough, so to speak zero approximation, the specific binding energy is constant and equal to approximately 8 MeV per
nucleon. The approximate independence of the specific binding energy from the number of nucleons indicates the saturation property of nuclear forces. This property is that each nucleon can only interact with a few neighboring nucleons.
b) The specific binding energy is not strictly constant, but has a maximum (~8.7 MeV/nucleon) at BUT= 56, i.e. in the area of iron nuclei, and falls to both edges. The maximum of the curve corresponds to the most stable nuclei. It is energetically advantageous for the lightest nuclei to merge with each other with the release of thermonuclear energy. For the heaviest nuclei, on the contrary, the process of fission into fragments is beneficial, which proceeds with the release of energy, called atomic energy.