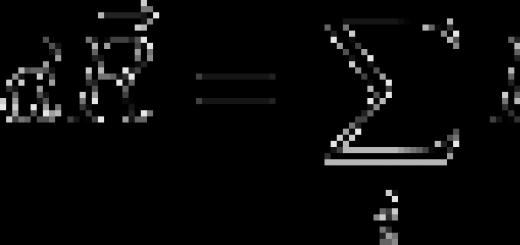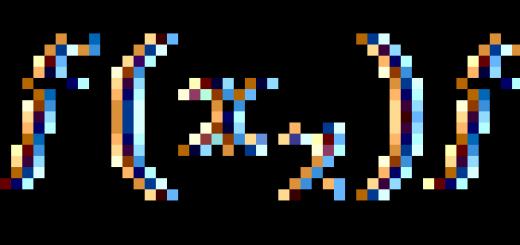"The properties of the elements, and therefore the simple and complex bodies (substances) formed by them, stand in a periodic dependence on their atomic weight."
Modern wording:
"the properties of chemical elements (i.e., the properties and form of the compounds they form) are in a periodic dependence on the charge of the nucleus of atoms of chemical elements."
The physical meaning of chemical periodicity
Periodic changes in the properties of chemical elements are due to the correct repetition of the electronic configuration of the external energy level (valence electrons) of their atoms with an increase in the nuclear charge.
Graphic image The periodic law is the periodic table. It contains 7 periods and 8 groups.
Period - horizontal rows of elements with the same maximum value of the main quantum number of valence electrons.
The period number indicates the number energy levels in the element atom.
Periods can consist of 2 (first), 8 (second and third), 18 (fourth and fifth), or 32 (sixth) elements, depending on the number of electrons in the outer energy level. The last, seventh period is incomplete.
All periods (except the first) begin with an alkali metal ( s- element) and end with a noble gas ( ns 2 np 6 ).
Metallic properties are considered as the ability of atoms of elements to easily donate electrons, and non-metallic properties to accept electrons due to the tendency of atoms to acquire a stable configuration with filled sublevels. Filling the outer s- sublevel indicates the metallic properties of the atom, and the formation of the outer p- sublevel - on non-metallic properties. An increase in the number of electrons by p- sublevel (from 1 to 5) enhances the non-metallic properties of the atom. Atoms with a fully formed, energetically stable configuration of the outer electron layer ( ns 2 np 6 ) chemically inert.
In long periods, the transition of properties from the active metal to the noble gas occurs more smoothly than in short periods, because the formation of an internal n - 1) d - sublevel while maintaining the external ns 2 - layer. Large periods consist of even and odd rows.
For elements of even rows on the outer layer ns 2 - electrons, therefore, metallic properties predominate and their weakening with increasing nuclear charge is small; in odd rows is formed np- sublevel, which explains the significant weakening of the metallic properties.
Groups - vertical columns of elements with the same number of valence electrons, equal to the group number. There are main and secondary subgroups.
The main subgroups consist of elements of small and large periods, the valence electrons of which are located on the outer ns - and np - sublevels.
Secondary subgroups consist of elements of only large periods. Their valence electrons are on the outer ns- sublevel and internal ( n - 1) d - sublevel (or (n - 2) f - sublevel).
Depending on which sublevel ( s-, p-, d- or f-) filled with valence electrons, the elements of the periodic system are divided into: s- elements (elements of the main subgroup I and II groups), p - elements (elements of the main subgroups III - VII groups), d - elements (elements of secondary subgroups), f- elements (lanthanides, actinides).
In the main subgroups, from top to bottom, metallic properties are enhanced, while non-metallic properties are weakened. The elements of the main and secondary groups differ greatly in properties.
The group number indicates the highest valency of the element (except O , F , elements of the copper subgroup and the eighth group).
Common to the elements of the main and secondary subgroups are the formulas of higher oxides (and their hydrates). For higher oxides and their element hydrates I-III groups (except for boron) the basic properties predominate, with IV to VIII - acidic.
The concept of elements as primary substances came from ancient times and, gradually changing and being refined, has come down to our time. The founders of scientific views on chemical elements are R. Boyle (7th century), M. V. Lomonosov (18th century) and Dalton (19th century).
To early XIX in. about 30 elements were known, by the middle of the 19th century - about 60. As the number of elements accumulated, the task of their systematization arose. Such attempts to D.I. Mendeleev was at least fifty; The systematization was based on: and atomic weight(now called atomic mass), and chemical equivalent, and valency. Approaching the classification of chemical elements metaphysically, trying to systematize only the elements known at that time, none of the predecessors of D. I. Mendeleev could discover the universal interconnection of elements, create a single harmonious system that reflects the law of development of matter. This important task for science was brilliantly solved in 1869 by the great Russian scientist D. I. Mendeleev, who discovered the periodic law.
Mendeleev took as the basis for systematization: a) atomic weight and b) chemical similarity between elements. The most striking, exponent of the similarity of the properties of elements is their same higher valency. Like atomic weight ( atomic mass), and the highest valency of the element are quantitative, numerical constants, convenient for systematization.
Arranging all the 63 elements known at that time in a row in order of increasing atomic masses, Mendeleev noticed the periodic repetition of the properties of elements at unequal intervals. As a result, Mendeleev created the first version of the periodic system.
The regular nature of the change in the atomic masses of the elements along the verticals and horizontals of the table, as well as the empty spaces formed in it, allowed Mendeleev to boldly predict the presence in nature of a number of elements that were not yet known to science at that time and even outline their atomic masses and basic properties, based on the assumed position elements in the table. This could be done only on the basis of a system that objectively reflects the law of development of matter. The essence of the periodic law was formulated by D. I. Mendeleev in 1869: “The properties of simple bodies, as well as the shapes and properties of compounds of elements, are in a periodic dependence on the value atomic weights(mass) elements".
Periodic system elements.
In 1871, D. I. Mendeleev gives the second version of the periodic system (the so-called short form table), in which he reveals the various degrees of relationship between the elements. This version of the system made it possible for Mendeleev to predict the existence of 12 elements and describe the properties of three of them with very high accuracy. Between 1875 and 1886 these three elements were discovered and a complete coincidence of their properties with those predicted by the great Russian scientist was revealed. These elements received the following names: scandium, gallium, germanium. After that, the periodic law received universal recognition as an objective law of nature and is now the foundation of chemistry, physics and other natural sciences.
The periodic system of chemical elements is a graphical expression of the periodic law. It is known that a number of laws, in addition to verbal formulations, can be depicted graphically and expressed mathematical formulas. Such is the periodic law; only the mathematical regularities inherent in it, which will be discussed below, are not yet united by a general formula. Knowledge of the periodic system facilitates the study of the course general chemistry.
The design of the modern periodic system, in principle, differs little from the version of 1871. The symbols of the elements in the periodic system are arranged in vertical and horizontal columns. This leads to the unification of elements into groups, subgroups, periods. Each element occupies a certain cell in the table. Vertical graphs are groups (and subgroups), horizontal graphs are periods (and series).
group called a set of elements with the same valency in oxygen. This highest valence is determined by the group number. Since the sum of the higher valences for oxygen and hydrogen for non-metal elements is eight, it is easy to determine the formula of the highest by the group number. hydrogen compound. So, for phosphorus - an element of the fifth group - the highest valence in oxygen is five, the formula of the highest oxide is P2O5, and the formula of the compound with hydrogen is PH3. For sulfur, an element of the sixth group, the formula of the highest oxide is SO3, and the highest compound with hydrogen is H2S.
Some elements have a higher valency that is not equal to the number of their groups. Such exceptions are copper Cu, silver Ag, gold Au. They are in the first group, but their valencies vary from one to three. For example, there are compounds: CuO; AgO; Cu2O3; Au2O3. Oxygen is placed in the sixth group, although its compounds with a valency higher than two are almost never found. Fluorine P - an element of group VII - is monovalent in its most important compounds; bromine Br - an element of group VII - is maximally pentavalent. There are especially many exceptions in group VIII. There are only two elements in it: ruthenium Ru and osmium Os exhibit a valency of eight, their higher oxides have the formulas RuO4 and OsO4. The valency of the remaining elements of group VIII is much lower.
Initially, Mendeleev's periodic system consisted of eight groups. At the end of the XIX century. inert elements were discovered, predicted by the Russian scientist N. A. Morozov, and the periodic system was replenished with the ninth group in a row - zero in number. Now many scientists consider it necessary to return to the division of all elements again into 8 groups. This makes the system more slender; From the positions of the octet (eight) groups, some rules and laws become clearer.
The elements of the group are distributed according to subgroups. A subgroup combines elements of a given group that are more similar in their chemical properties. This similarity depends on the analogy in the structure of the electron shells of the atoms of the elements. In the periodic system, the symbols of the elements of each of the subgroups are placed strictly vertically.
In the first seven groups, there is one main and one secondary subgroup; in the eighth group there is one main subgroup, "inert" elements, and three secondary ones. The name of each subgroup is usually given by the name of the top element, for example: lithium subgroup (Li-Na-K-Rb-Cs-Fr), chromium subgroup (Cr-Mo-W). While the elements of the same subgroup are chemical analogues, elements of different subgroups of the same group sometimes differ very sharply in their properties. common property for the elements of the main and secondary subgroups of the same group, there is basically only the same highest valency for oxygen. So, manganese Mn and chlorine C1, which are in different subgroups of group VII, chemically have almost nothing in common: manganese is a metal, chlorine is a typical non-metal. However, the formulas of their higher oxides and the corresponding hydroxides are similar: Mn2O7 - Cl2O7; HMnO4 - HC1O4.
In the periodic table, there are two horizontal rows of 14 elements located outside the groups. Usually they are placed at the bottom of the table. One of these rows consists of elements called lanthanides (literally: similar to lanthanum), the other row - elements of actinides (similar to actinium). The actinide symbols are located below the lanthanide symbols. This arrangement reveals 14 shorter subgroups, each consisting of 2 elements: these are the second side, or lanthanide-actinide subgroups.
On the basis of what has been said, there are: a) main subgroups, b) side subgroups and c) second side (lanthanide-actinide) subgroups.
It should be noted that some of the main subgroups also differ from each other in the structure of the atoms of their elements. Based on this, all subgroups of the periodic system can be divided into 4 categories.
I. Main subgroups of groups I and II (lithium and beryllium subgroups).
II. Six main subgroups III - IV - V - VI - VII - VIII groups (subgroups of boron, carbon, nitrogen, oxygen, fluorine and neon).
III. Ten secondary subgroups (one each in groups I-VII and three in group VIII). jfc,
IV. Fourteen lanthanide-actinide subgroups.
The number of subgroups of these 4 categories is an arithmetic progression: 2-6-10-14.
It should be noted that the top element of any main subgroup is in period 2; the upper element of any side - in the 4th period; the top element of any lanthanide-actinide subgroup is in the 6th period. Thus, with each new even period of the periodic system, new categories of subgroups appear.
Each element, except for being in a particular group and subgroup, is also in one of the seven periods.
A period is such a sequence of elements, during which their properties change in order of gradual strengthening from typically metallic to typically non-metallic (metalloid). Each period ends with an inert element. As the metallic properties are weakened, non-metallic properties begin to appear in the elements and gradually increase; in the middle of the periods there are usually elements that combine, to one degree or another, both metallic and non-metallic properties. These elements are often called amphoteric.
The composition of the periods.
Periods are not uniform in the number of elements included in them. The first three are called small, the other four are called large. On fig. 8 shows the composition of the periods. The number of elements in any period is expressed by the formula 2p2 where n is an integer. In periods 2 and 3 there are 8 elements each; in 4 and 5 - 18 elements each; in 6-32 elements; in 7, not yet finished, there are 18 elements, although theoretically there should also be 32 elements.
Original 1 period. It contains only two elements: hydrogen H and helium He. The transition of properties from metallic to non-metallic takes place: here in one typically amphoteric element - hydrogen. The latter, according to some metallic properties inherent in it, leads the subgroup of alkali metals, according to its non-metallic properties, it leads the subgroup of halogens. Hydrogen is therefore often placed in the periodic system twice - in groups 1 and 7.
The different quantitative composition of the periods leads to an important consequence: neighboring elements of small periods, for example, carbon C and nitrogen N, differ sharply from each other in their properties, while neighboring elements of large periods, for example, lead Pb and bismuth Bi, are much closer in properties to each other. to each other, since the change in the nature of the elements in large periods occurs in small jumps. In separate sections of long periods, even such a slow decline in metallicity is observed that adjacent elements turn out to be very similar in their chemical properties. Such, for example, is the triad of elements of the fourth period: iron Fe - cobalt Co - nickel Ni, which is often called the "iron family". Horizontal similarity (horizontal analogy) overlaps here even vertical similarity (vertical analogy); Thus, the elements of the iron subgroup - iron, ruthenium, osmium - are less chemically similar to each other than the elements of the "iron family".
Most a prime example the horizontal analogy are the lanthanides. All of them are chemically similar to each other and to lanthanum La. In nature, they are found in companies, it is difficult to separate, the typical highest valency of most of them is 3. A special internal periodicity has been found in lanthanides: every eighth of them, in order of arrangement, repeats to some extent the properties and valence states of the first, i.e. the one from which the counting starts. Thus, terbium Tb is similar to cerium Ce; lutetium Lu - to gadolinium Gd.
Actinides are similar to lanthanides, but their horizontal analogy is manifested to a much lesser extent. The highest valency of some actinides (for example, uranium U) reaches six. Fundamentally possible and among them internal periodicity has not yet been confirmed.
Arrangement of elements in the periodic system. Moseley's law.
D. I. Mendeleev arranged the elements in a certain sequence, sometimes called the "Mendeleev series". In general, this sequence (numbering) is associated with an increase in the atomic masses of the elements. However, there are exceptions. Sometimes the logical course of the change in valence is in conflict with the course of the change in atomic masses In such cases, the need required to give preference to any one of these two bases of systematization. In some cases, D. I. Mendeleev violated the principle of the arrangement of elements according to increasing atomic masses and relied on the chemical analogy between the elements. If Mendeleev had placed nickel Ni before cobalt Co, iodine I before Te tellurium, then these elements would fall into subgroups and groups that do not correspond to their properties and their highest valency.
In 1913, the English scientist G. Moseley, studying the spectra of X-rays for various elements, noticed a pattern connecting the numbers of elements in the periodic system of Mendeleev with the wavelength of these rays, resulting from the irradiation of certain elements with cathode clouds. It turned out that square roots from the reciprocal values of the wavelengths of these rays are linearly related to the serial numbers of the corresponding elements. G. Moseley's law made it possible to verify the correctness of the "Mendeleev series" and confirmed its impeccability.
Let, for example, the values for elements No. 20 and No. 30 are known, the numbers of which in the system do not cause us doubts. These values are related to the specified numbers in a linear relationship. To check, for example, the correctness of the number assigned to cobalt (27), and judging by the atomic mass, nickel should have had this number, it is irradiated with cathode rays: as a result, X-rays are emitted from cobalt. Decomposing them into suitable gratings(on crystals) we obtain the spectrum of these rays and, having chosen the clearest of the spectral lines, we measure the wavelength () of the beam corresponding to this line; then set aside the value on the ordinate. From the obtained point A, we draw a straight line parallel to the x-axis, until it intersects with the previously identified straight line. From the point of intersection B, we lower the perpendicular to the abscissa axis: it will accurately indicate to us the number of cobalt equal to 27. So, the periodic system of elements of D. I. Mendeleev - the fruit of the scientist's logical conclusions - received experimental confirmation.
The modern formulation of the periodic law. physical meaning the ordinal number of the element.
After the work of G. Moseley, the atomic mass of an element gradually began to give way to its leading role to a new, not yet clear in its internal (physical) meaning, but a clearer constant - the ordinal or, as they are now called, the atomic number of the element. The physical meaning of this constant was revealed in 1920 by the work of the English scientist D. Chadwick. D. Chadwick experimentally established that the ordinal number of an element is numerically equal to the value of the positive charge Z of the atomic nucleus of this element, i.e., the number of protons in the nucleus. It turned out that D. I. Mendeleev, without suspecting it, arranged the elements in a sequence exactly corresponding to the increase in the charge of the nuclei of their atoms.
By the same time, it was also established that atoms of the same element can differ from each other in their mass; such atoms are called isotopes. Atoms can serve as an example: and . In the periodic table, isotopes of the same element occupy one cell. In connection with the discovery of isotopes, the concept of a chemical element was clarified. Currently chemical element called the type of atoms that have the same nuclear charge - the same number of protons in the nucleus. The formulation of the periodic law was also refined. The modern formulation of the law says: the properties of elements and their compounds are in a periodic dependence on the size, charge of the nuclei of their atoms.
Other characteristics of the elements associated with the structure of the outer electronic layers of atoms, atomic volumes, ionization energy and other properties also change periodically.
Periodic system and structure of electron shells of atoms of elements.
Later it was found that not only the serial number of the element has a deep physical meaning, but also other concepts previously considered earlier also gradually acquired a physical meaning. For example, the group number, indicating the highest valency of the element, thereby reveals the maximum number of electrons of an atom of a particular element that can participate in the formation chemical bond.
The period number, in turn, turned out to be related to the number of energy levels present in the electron shell of an atom of an element of a given period.
Thus, for example, the "coordinates" of tin Sn (serial number 50, period 5, main subgroup of group IV) mean that there are 50 electrons in the tin atom, they are distributed over 5 energy levels, only 4 electrons are valence.
The physical meaning of finding elements in subgroups of various categories is extremely important. It turns out that for elements located in subgroups of category I, the next (last) electron is located on the s-sublevel of the outer level. These elements belong to the electronic family. For atoms of elements located in subgroups of category II, the next electron is located on the p-sublevel of the outer level. These are the elements of the “p” electronic family. Thus, the next 50th electron of tin atoms is located on the p-sublevel of the outer, i.e., 5th energy level.
For atoms of elements of subgroups of category III, the next electron is located on the d-sublevel, but already before the outer level, these are elements of the electronic family "d". For lanthanide and actinide atoms, the next electron is located on the f-sublevel, before the external level. These are the elements of the electronic family "f".
It is no coincidence, therefore, that the numbers of subgroups of these 4 categories noted above, that is, 2-6-10-14, coincide with the maximum numbers of electrons in the s-p-d-f sublevels.
But it turns out that it is possible to solve the problem of the order of filling the electron shell and derive an electronic formula for an atom of any element and on the basis of the periodic system, which clearly indicates the level and sublevel of each successive electron. The periodic system also indicates the placement of elements one after another into periods, groups, subgroups and the distribution of their electrons by levels and sublevels, because each element has its own, characterizing its last electron. As an example, let us analyze the compilation of an electronic formula for the atom of the element zirconium (Zr). The periodic system gives the indicators and "coordinates" of this element: serial number 40, period 5, group IV, side subgroup. First conclusions: a) all 40 electrons, b) these 40 electrons are distributed over five energy levels; c) out of 40 electrons only 4 are valence, d) the next 40th electron entered the d-sublevel before the outer, i.e. the fourth energy level.Similar conclusions can be drawn about each of the 39 elements preceding zirconium, only the indicators and coordinates will be different each time.
Therefore, the methodical method of compiling the electronic formulas of elements based on the periodic system consists in the fact that we sequentially consider the electron shell of each element along the path to the given one, identifying by its “coordinates” where its next electron went in the shell.
The first two elements of the first period, hydrogen H and helium, do not belong to the s-family. Two of their electrons go to the s-sublevel of the first level. We write down: The first period ends here, the first energy level also. The next two elements of the second period, lithium Li and beryllium Be, are in the main subgroups of groups I and II. These are also s-elements. Their next electrons will be located on the s sublevel of the 2nd level. We write down Next, 6 elements of the 2nd period follow in a row: boron B, carbon C, nitrogen N, oxygen O, fluorine F and neon Ne. According to the location of these elements in the main subgroups of the III - Vl groups, their next six electrons will be located on the p-sublevel of the 2nd level. We write down: The second period ends with the inert element neon, the second energy level is also completed. This is followed by two elements of the third period of the main subgroups of groups I and II: sodium Na and magnesium Mg. These are s-elements and their next electrons are located on the s-sublevel of the 3rd level. Then there are six elements of the 3rd period: aluminum Al, silicon Si, phosphorus P, sulfur S, chlorine C1, argon Ar. According to the location of these elements in the main subgroups of groups III - VI, their next electrons, among six, will be located on the p-sublevel of the 3rd level - The 3rd period is completed by the inert element argon, but the 3rd energy level is not yet completed, while there are no electrons on its third possible d-sublevel.
This is followed by 2 elements of the 4th period of the main subgroups of groups I and II: potassium K and calcium Ca. These are again s-elements. Their next electrons will be at the s-sublevel, but already at the 4th level. It is energetically more profitable for these next electrons to start filling the 4th level, which is more distant from the nucleus, than to fill the 3d sublevel. We write down: The following ten elements of the 4th period from No. 21 scandium Sc to No. 30 zinc Zn are in side subgroups III - V - VI - VII - VIII - I - II groups. Since they are all d-elements, their next electrons are located on the d-sublevel before the outer level, i.e., the third from the nucleus. We write down:
The following six elements of the 4th period: gallium Ga, germanium Ge, arsenic As, selenium Se, bromine Br, krypton Kr - are in the main subgroups III - VIIJ of groups. Their next 6 electrons are located on the p-sublevel of the outer, i.e., 4th level: 3b elements are considered; the fourth period is completed by the inert element krypton; completed and the 3rd energy level. However, at level 4, only two sublevels are completely filled: s and p (out of 4 possible).
This is followed by 2 elements of the 5th period of the main subgroups of I and II groups: No. 37 rubidium Rb and No. 38 strontium Sr. These are elements of the s-family, and their next electrons are located on the s-sublevel of the 5th level: The last 2 elements - No. 39 yttrium YU No. 40 zirconium Zr - are already in side subgroups, i.e., belong to the d-family. Two of their next electrons will go to the d-sublevel, before the outer, i.e. Level 4 Summing up all the entries in succession, we compose the electronic formula for the zirconium atom No. 40 The derived electronic formula for the zirconium atom can be slightly modified by arranging the sublevels in the order of numbering their levels:
The derived formula can, of course, be simplified into the distribution of electrons only over energy levels: Zr – 2|8| 18 |8 + 2| 2 (the arrow indicates the entry point of the next electron; valence electrons are underlined). The physical meaning of the category of subgroups lies not only in the difference in the place where the next electron enters the shell of the atom, but also in the levels at which the valence electrons are located. From a comparison of simplified electronic formulas, for example, chlorine (3rd period, main subgroup of group VII), zirconium (5th period, secondary subgroup of group IV) and uranium (7th period, lanthanide-actinide subgroup)
№17, С1-2|8|7
№40, Zr - 2|8|18|8+ 2| 2
№92, U - 2|8|18 | 32 |18 + 3|8 + 1|2
it can be seen that for elements of any main subgroup, only electrons of the outer level (s and p) can be valence. For elements of secondary subgroups, electrons of the outer and partially pre-external level (s and d) can be valence. In lanthanides and especially actinides, valence electrons can be located at three levels: external, pre-external, and pre-external. Usually, total number valence electrons is equal to the group number.
Element properties. Ionization energy. Electron affinity energy.
A comparative consideration of the properties of elements is carried out in three possible directions of the periodic system: a) horizontal (by period), b) vertical (by subgroup), c) diagonal. To simplify the reasoning, we exclude the 1st period, the unfinished 7th, as well as the entire VIII group. The main parallelogram of the system will remain, in the upper left corner of which there will be lithium Li (No. 3), in the lower left corner - cesium Cs (No. 55). In the upper right - fluorine F (No. 9), in the lower right - astatine Аt (No. 85).
directions. In the horizontal direction from left to right, the volumes of atoms gradually decrease; occurs, this is a result of the influence of an increase in the charge of the nucleus on the electron shell. In the vertical direction from top to bottom, as a result of an increase in the number of levels, the volumes of atoms gradually increase; in the diagonal direction - much less distinctly expressed and shorter - remain close. These are general patterns, of which, as always, there are exceptions.
In the main subgroups, as the volumes of atoms increase, i.e., from top to bottom, the elimination of external electrons becomes easier and the addition of new electrons to atoms becomes more difficult. The recoil of electrons characterizes the so-called reducing ability of elements, which is especially typical for metals. The addition of electrons characterizes the oxidizing ability, which is typical for non-metals. Consequently, from top to bottom in the main subgroups, the reducing power of the atoms of the elements increases; the metallic properties of simple bodies corresponding to these elements also increase. The oxidative capacity is reduced.
From left to right, according to the periods, the picture of changes is opposite: the reducing ability of the atoms of the elements decreases, while the oxidizing one increases; the non-metallic properties of simple bodies corresponding to these elements increase.
In the diagonal direction, the properties of the elements remain more or less close. Consider this direction on an example: beryllium-aluminum  From beryllium Be to aluminum Al, one can go directly along the diagonal Be → A1, it is also possible through boron B, i.e., along two legs Be → B and B → A1. The strengthening of non-metallic properties from beryllium to boron and their weakening from boron to aluminum explains why the elements beryllium and aluminum, located diagonally, have some analogy in properties, although they are not in the same subgroup of the periodic table.
From beryllium Be to aluminum Al, one can go directly along the diagonal Be → A1, it is also possible through boron B, i.e., along two legs Be → B and B → A1. The strengthening of non-metallic properties from beryllium to boron and their weakening from boron to aluminum explains why the elements beryllium and aluminum, located diagonally, have some analogy in properties, although they are not in the same subgroup of the periodic table.
Thus, between the periodic system, the structure of the atoms of the elements and their chemical properties there is a close relationship.
The properties of an atom of any element - to donate an electron and turn into a positively charged ion - are quantified by the expenditure of energy, called the ionization energy I*. It is expressed in kcal/g-atom or hJ/g-atom.
![]()
The lower this energy, the stronger the atom of the element exhibits reducing properties, the more metallic the element; the more this energy, the weaker the metallic properties, the stronger the non-metallic properties of the element. The property of an atom of any element to accept an electron and at the same time turn into a negatively charged ion is estimated by the amount of energy released, called more energetic electron affinity E; it is also expressed in kcal/g-atom or kJ/g-atom.
![]()
Electron affinity can serve as a measure of an element's ability to exhibit non-metallic properties. The greater this energy, the more non-metallic the element, and, conversely, the lower the energy, the more metallic the element.
Often, to characterize the properties of elements, a value is used, which is called electronegativity.
She: represents arithmetic sum ionization energy and electron affinity energy
The constant is a measure of the non-metallicity of elements. The larger it is, the stronger the element exhibits non-metallic properties.
It should be borne in mind that all elements are essentially dual in nature. The division of elements into metals and non-metals is, to a certain extent, conditional, because there are no sharp edges in nature. With an increase in the metallic properties of an element, its non-metaglic properties are weakened and vice versa. The most "metallic" of the elements - francium Fr - can be considered the least non-metallic, the most "non-metallic" - fluorine F - can be considered the least metallic.
Summing up the values of the calculated energies - ionization energy and electron affinity energy - we get: for cesium the value is 90 kcal/g-a., for lithium 128 kcal/g-a., for fluorine = 510 kcal/g-a. (The value is also expressed in kJ/g-a.). These are the absolute values of electronegativity. To simplify, use relative values electronegativity, taking the electronegativity of lithium (128) as unity. Then for fluorine (F) we get:

For cesium (Cs), the relative electronegativity will be
On the graph of changes in the electronegativity of the elements of the main subgroups
I-VII groups. the electronegativity of the elements of the main subgroups of groups I-VII was compared. The given data indicate the true position of hydrogen in the 1st period; unequal increase in the metallicity of the elements, from top to bottom in various subgroups; some similarity of elements: hydrogen - phosphorus - tellurium (= 2.1), beryllium and aluminum (= 1.5) and a number of other elements. As can be seen from the above comparisons, using the values of electronegativity, it is possible to approximately compare among themselves, elements of even different subgroups, and different periods.

Graph of changes in the electronegativity of the elements of the main subgroups of groups I-VII.
The periodic law and the periodic system of elements are of great philosophical, scientific and methodological significance. They are: a means of knowing the world around us. The periodic law reveals and reflects the dialectical-materialistic essence of nature. Periodic law and the periodic system of elements convincingly prove the unity and materiality of the world around us. They are the best confirmation of the validity of the main features of the Marxist dialectical method of cognition: a) the interconnection and interdependence of objects and phenomena, b) the continuity of movement and development, c) the transition of quantitative changes into qualitative ones, d) the struggle and unity of opposites.
Huge scientific significance The periodic law lies in the fact that it helps creative discoveries in the field of chemical, physical, mineralogical, geological, technical and other sciences. Before the discovery of the periodic law, chemistry was an accumulation of isolated, factual information devoid of internal connection; now all this is brought into a single coherent system. Many discoveries in the field of chemistry and physics were made on the basis of the periodic law and the periodic table of elements. The periodic law opened the way to understanding the internal structure of the atom and its nucleus. It is enriched with new discoveries and is confirmed as an unshakable, objective law of nature. The great methodological and methodological significance of the periodic law and the periodic system of elements lies in the fact that when studying chemistry they provide an opportunity to develop a student's dialectical materialistic worldview and facilitate the assimilation of a chemistry course: The study of chemistry should not be based on memorizing the properties of individual elements and their compounds, but judge the properties of simple and complex substances, based on the patterns expressed by the periodic law and the periodic system of elements.
IV - VII - big periods, because consist of two rows (even and odd) of elements.
In even rows of large periods are located typical metals. The odd series begins with a metal, then the metallic properties weaken and the non-metallic properties increase, the period ends with an inert gas.
Group is a vertical row of chem. elements combined by chem. properties.
Group
main subgroup secondary subgroup
The main subgroup includes The secondary subgroup includes
elements of both small and large elements of only large periods.
periods.
![]()
![]() H, Li, Na, K, Rb, Cs, Fr Cu, Ag, Au
H, Li, Na, K, Rb, Cs, Fr Cu, Ag, Au
small big big
For elements combined in the same group, the following patterns are characteristic:
1. Highest valency of elements in compounds with oxygen(with a few exceptions) corresponds to the group number.
Elements of secondary subgroups may also exhibit another higher valency. For example, Cu - an element of group I of the side subgroup - forms oxide Cu 2 O. However, the most common are compounds of divalent copper.
2. In the main subgroups(top down) with an increase in atomic masses, the metallic properties of the elements increase and the non-metallic ones weaken.
The structure of the atom.
For a long time, science was dominated by the opinion that atoms are indivisible, i.e. do not contain simpler components.
However, at the end of the 19th century, a number of facts were established that testified to complex composition atoms and the possibility of their mutual transformations.
Atoms are complex formations built from smaller structural units.
|
|
ē - electron - outside the nucleus
For chemistry, the structure of the electron shell of the atom is of great interest. Under electron shell understand the totality of all electrons in an atom. The number of electrons in an atom is equal to the number of protons, i.e. the atomic number of the element, since the atom is electrically neutral.
The most important characteristic of an electron is the energy of its bond with an atom. Electrons with similar energy values form a single electronic layer.
Each chem. element in the periodic table was numbered.
The number that each element receives is called serial number.
The physical meaning of the serial number:
1. What is the serial number of the element, such is the charge of the nucleus of the atom.
2. The same number of electrons revolve around the nucleus.
Z = p + Z - element number

n 0 \u003d A - Z
n 0 \u003d A - p + A - atomic mass of the element
n 0 \u003d A - ē
For example Li.
The physical meaning of the period number.
In what period is the element, how many electron shells (layers) it will have.
| |

 Not +2
Not +2 | |
| |
Determination of the maximum number of electrons in one electron shell.
1. Specify the name of the element, its designation. Determine the element's serial number, period number, group, subgroup. Indicate the physical meaning of the system parameters - serial number, period number, group number. Justify the position in the subgroup.
2. Indicate the number of electrons, protons and neutrons in an atom of an element, nuclear charge, mass number.
3. Make a complete electronic formula of the element, determine the electronic family, assign a simple substance to the class of metals or non-metals.
4. Draw graphically the electronic structure of the element (or the last two levels).
5. Graphically depict all possible valence states.
6. Specify the number and type of valence electrons.
7. List all possible valencies and oxidation states.
8. Write the formulas of oxides and hydroxides for all valence states. Indicate their chemical nature (confirm the answer with the equations of the corresponding reactions).
9. Give the formula of a hydrogen compound.
10. Name the scope of this element
Decision. Scandium corresponds to the element with the atomic number 21 in the PSE.
1. The element is in the IV period. The period number means the number of energy levels in the atom of this element, it has 4 of them. Scandium is located in the 3rd group - on the outer level of the 3rd electron; in the side group. Therefore, its valence electrons are in the 4s and 3d sublevels. The serial number numerically coincides with the charge of the nucleus of an atom.
2. The charge of the nucleus of the scandium atom is +21.
The number of protons and electrons is 21 each.
The number of neutrons A–Z = 45 – 21 = 24.
The total composition of the atom: ( ![]() ).
).
3. Full electronic formula of scandium:
1s 2 2s 2 2p 6 3s 2 3p 6 3d 1 4s 2 .
Electron family: d-element, as in the process of filling
d-orbitals. Electronic structure atom ends with s-electrons, so scandium exhibits metallic properties; simple substance - metal.
4. Electronic graphic configuration looks like:

5. Possible valence states due to the number of unpaired electrons:
- in basic condition:
– in scandium in an excited state, an electron from the 4s orbital will move to a free 4p orbital, one unpaired d-electron increases valence possibilities scandium.
Sc has three valence electrons in the excited state.
6. Possible valencies in this case are determined by the number of unpaired electrons: 1, 2, 3 (or I, II, III). Possible oxidation states (reflecting the number of displaced electrons) +1, +2, +3 (since scandium is a metal).
7. The most characteristic and stable valency III, oxidation state +3. The presence of only one electron in the d state is responsible for the low stability of the 3d 1 4s 2 configuration.
Scandium and its analogues, unlike other d-elements, exhibits a constant oxidation state of +3, this highest degree oxidation and corresponds to the group number.
8. Formulas of oxides and their chemical nature:
form of higher oxide - (amphoteric);
hydroxide formulas: – amphoteric.
Reaction equations confirming the amphoteric nature of oxides and hydroxides:
![]() (scandate of lithium),
(scandate of lithium),
(scandium chloride),
( potassium hexahydroxoscandiate (III) ),
(scandium sulfate).
9. It does not form compounds with hydrogen, since it is in the side subgroup and is a d-element.
10. Scandium compounds are used in semiconductor technology.
Example 2 Which of the two elements, manganese or bromine, has more pronounced metallic properties?
Decision. These elements are in the fourth period. We write down their electronic formulas:
Manganese is a d-element, i.e. an element of a side subgroup, and bromine is
p-element of the main subgroup of the same group. At the outer electronic level, the manganese atom has only two electrons, while the bromine atom has seven. The radius of the manganese atom is less than the radius of the bromine atom with the same number of electron shells.
A common pattern for all groups containing p- and d-elements is the predominance of metallic properties in d-elements.
Thus, the metallic properties of manganese are more pronounced than those of bromine.