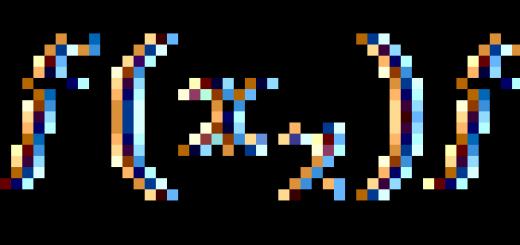Chemicals are the things that make up the world around us.
The properties of each chemical substance are divided into two types: these are chemical, which characterize its ability to form other substances, and physical, which are objectively observed and can be considered in isolation from chemical transformations. For example, the physical properties of a substance are its state of aggregation(solid, liquid or gaseous), thermal conductivity, heat capacity, solubility in various environments(water, alcohol, etc.), density, color, taste, etc.
Transformations of some chemical substances into other substances are called chemical phenomena or chemical reactions. It should be noted that there are also physical phenomena, which, obviously, are accompanied by a change in some physical properties substances without being converted into other substances. To physical phenomena, for example, include the melting of ice, the freezing or evaporation of water, etc.
The fact that in the course of any process a chemical phenomenon takes place can be concluded by observing characteristics chemical reactions such as color change, precipitation, gas evolution, heat and/or light evolution.
So, for example, a conclusion about the course of chemical reactions can be made by observing:
The formation of sediment when boiling water, called scale in everyday life;
The release of heat and light during the burning of a fire;
Changing the color of a slice of a fresh apple in the air;
The formation of gas bubbles during the fermentation of dough, etc.
The smallest particles of matter, which in the process of chemical reactions practically do not undergo changes, but only in a new way are connected to each other, are called atoms.
The very idea of the existence of such units of matter arose in ancient greece in the minds of ancient philosophers, which actually explains the origin of the term "atom", since "atomos" literally translated from Greek means "indivisible".
However, contrary to the idea of the ancient Greek philosophers, atoms are not the absolute minimum of matter, i.e. themselves have a complex structure.
Each atom consists of the so-called subatomic particles - protons, neutrons and electrons, denoted respectively by the symbols p + , n o and e - . The superscript in the notation used indicates that the proton has a unit positive charge, the electron has a unit negative charge, and the neutron has no charge.
As for the qualitative structure of the atom, each atom has all the protons and neutrons concentrated in the so-called nucleus, around which the electrons form an electron shell.
The proton and neutron have practically the same masses, i.e. m p ≈ m n , and the electron mass is almost 2000 times less than the mass of each of them, i.e. m p / m e ≈ m n / m e ≈ 2000.
Insofar as fundamental property of an atom is its electrical neutrality, and the charge of one electron is equal to the charge of one proton, from this we can conclude that the number of electrons in any atom is equal to the number of protons.
So, for example, the table below shows the possible composition of atoms:
The type of atoms with the same nuclear charge, i.e. with the same number of protons in their nuclei is called a chemical element. Thus, from the table above, we can conclude that atom1 and atom2 belong to one chemical element, and atom3 and atom4 belong to another chemical element.
Each chemical element has its own name and individual symbol, which is read in a certain way. So, for example, the simplest chemical element, the atoms of which contain only one proton in the nucleus, has the name "hydrogen" and is denoted by the symbol "H", which is read as "ash", and the chemical element with a nuclear charge of +7 (i.e. containing 7 protons) - "nitrogen", has the symbol "N", which is read as "en".
As you can see from the table above, the atoms of one chemical element may differ in the number of neutrons in the nuclei.
Atoms belonging to the same chemical element, but having a different number of neutrons and, as a result, mass, are called isotopes.
So, for example, the chemical element hydrogen has three isotopes - 1 H, 2 H and 3 H. The indices 1, 2 and 3 above the H symbol mean the total number of neutrons and protons. Those. knowing that hydrogen is a chemical element, which is characterized by the fact that there is one proton in the nuclei of its atoms, we can conclude that there are no neutrons at all in the 1 H isotope (1-1 = 0), in the 2 H isotope - 1 neutron (2-1=1) and in the isotope 3 H - two neutrons (3-1=2). Since, as already mentioned, a neutron and a proton have the same masses, and the mass of an electron is negligible compared to them, this means that the 2 H isotope is almost twice as heavy as the 1 H isotope, and the 3 H isotope is even three times as heavy. . In connection with such a large spread in the masses of hydrogen isotopes, the isotopes 2 H and 3 H were even assigned separate individual titles and symbols, which is not characteristic of any other chemical element. The 2 H isotope was named deuterium and given the symbol D, and the 3 H isotope was given the name tritium and given the symbol T.
If we take the mass of the proton and neutron as unity, and neglect the mass of the electron, in fact, the upper left index, in addition to the total number of protons and neutrons in the atom, can be considered its mass, and therefore this index is called the mass number and is denoted by the symbol A. Since the charge of the nucleus of any protons correspond to the atom, and the charge of each proton is conventionally considered equal to +1, the number of protons in the nucleus is called the charge number (Z). Denoting the number of neutrons in an atom with the letter N, mathematically the relationship between mass number, charge number and number of neutrons can be expressed as:
According to modern concepts, the electron has a dual (particle-wave) nature. It has the properties of both a particle and a wave. Like a particle, an electron has a mass and a charge, but at the same time, the flow of electrons, like a wave, is characterized by the ability to diffraction.
To describe the state of an electron in an atom, representations are used quantum mechanics, according to which the electron does not have a specific trajectory of motion and can be located at any point in space, but with different probabilities.
The region of space around the nucleus where an electron is most likely to be found is called the atomic orbital.
An atomic orbital can have a different shape, size and orientation. An atomic orbital is also called an electron cloud.
Graphically, one atomic orbital is usually denoted as a square cell:
Quantum mechanics has an extremely complex mathematical apparatus, therefore, within the framework of school course chemistry, only the consequences of quantum mechanical theory are considered.
According to these consequences, any atomic orbital and an electron located on it are completely characterized by 4 quantum numbers.
- The main quantum number - n - determines the total energy of an electron in a given orbital. The range of values of the main quantum number is all integers, i.e. n = 1,2,3,4, 5 etc.
- The orbital quantum number - l - characterizes the shape of the atomic orbital and can take any integer values from 0 to n-1, where n, recall, is the main quantum number.
Orbitals with l = 0 are called s-orbitals. s-orbitals are spherical and do not have a direction in space:
Orbitals with l = 1 are called p-orbitals. These orbitals have the shape of a three-dimensional figure eight, i.e. the shape obtained by rotating the figure eight around the axis of symmetry, and outwardly resemble a dumbbell:
Orbitals with l = 2 are called d-orbitals, and with l = 3 – f-orbitals. Their structure is much more complex.
3) The magnetic quantum number - m l - determines the spatial orientation of a particular atomic orbital and expresses the projection of the orbital angular momentum on the direction magnetic field. The magnetic quantum number m l corresponds to the orientation of the orbital relative to the direction of the external magnetic field strength vector and can take any integer values from –l to +l, including 0, i.e. the total number of possible values is (2l+1). So, for example, with l = 0 m l = 0 (one value), with l = 1 m l = -1, 0, +1 (three values), with l = 2 m l = -2, -1, 0, +1 , +2 (five values of the magnetic quantum number), etc.
So, for example, p-orbitals, i.e. orbitals with an orbital quantum number l = 1, having the shape of a “three-dimensional figure eight”, correspond to three values of the magnetic quantum number (-1, 0, +1), which, in turn, corresponds to three directions in space perpendicular to each other.
4) The spin quantum number (or simply spin) - m s - can be conditionally considered responsible for the direction of rotation of an electron in an atom, it can take on values. Electrons with different spins are indicated by vertical arrows pointing in different sides: ↓ and .
The set of all orbitals in an atom that have the same value of the principal quantum number is called the energy level or electron shell. Any arbitrary energy level with some number n consists of n 2 orbitals.
The set of orbitals with the same values of the principal quantum number and the orbital quantum number is an energy sublevel.
Each energy level, which corresponds to the main quantum number n, contains n sublevels. In turn, each energy sublevel with an orbital quantum number l consists of (2l+1) orbitals. Thus, the s-sublayer consists of one s-orbital, the p-sublayer - three p-orbitals, the d-sublayer - five d-orbitals, and the f-sublayer - seven f-orbitals. Since, as already mentioned, one atomic orbital is often denoted by one square cell, the s-, p-, d- and f-sublevels can be graphically depicted as follows:
Each orbital corresponds to an individual strictly defined set of three quantum numbers n, l and m l .
The distribution of electrons in orbitals is called the electron configuration.
The filling of atomic orbitals with electrons occurs in accordance with three conditions:
- The principle of minimum energy: Electrons fill orbitals starting from the lowest energy sublevel. The sequence of sublevels in order of increasing energy is as follows: 1s<2s<2p<3s<3p<4s≤3d<4p<5s≤4d<5p<6s…;
In order to make it easier to remember this sequence of filling electronic sublevels, the following graphic illustration is very convenient:
- Pauli principle: Each orbital can hold at most two electrons.
If there is one electron in the orbital, then it is called unpaired, and if there are two, then they are called an electron pair.
- Hund's rule: the most stable state of an atom is one in which, within one sublevel, the atom has the maximum possible number of unpaired electrons. This most stable state of the atom is called the ground state.
In fact, the above means that, for example, the placement of the 1st, 2nd, 3rd and 4th electrons on three orbitals of the p-sublevel will be carried out as follows:
The filling of atomic orbitals from hydrogen, which has a charge number of 1, to krypton (Kr) with a charge number of 36, will be carried out as follows:
A similar representation of the order in which atomic orbitals are filled is called an energy diagram. Based on the electronic diagrams of individual elements, you can write down their so-called electronic formulas (configurations). So, for example, an element with 15 protons and, as a result, 15 electrons, i.e. phosphorus (P) will have the following energy diagram:
When translated into an electronic formula, the phosphorus atom will take the form:
15 P = 1s 2 2s 2 2p 6 3s 2 3p 3
Normal-sized digits to the left of the sublevel symbol show the number of the energy level, and superscripts to the right of the sublevel symbol show the number of electrons in the corresponding sublevel.
Below are the electronic formulas of the first 36 elements of D.I. Mendeleev.
| period | Item No. | symbol | title | electronic formula |
| I | 1 | H | hydrogen | 1s 1 |
| 2 | He | helium | 1s2 | |
| II | 3 | Li | lithium | 1s2 2s1 |
| 4 | Be | beryllium | 1s2 2s2 | |
| 5 | B | boron | 1s 2 2s 2 2p 1 | |
| 6 | C | carbon | 1s 2 2s 2 2p 2 | |
| 7 | N | nitrogen | 1s 2 2s 2 2p 3 | |
| 8 | O | oxygen | 1s 2 2s 2 2p 4 | |
| 9 | F | fluorine | 1s 2 2s 2 2p 5 | |
| 10 | Ne | neon | 1s 2 2s 2 2p 6 | |
| III | 11 | Na | sodium | 1s 2 2s 2 2p 6 3s 1 |
| 12 | mg | magnesium | 1s 2 2s 2 2p 6 3s 2 | |
| 13 | Al | aluminum | 1s 2 2s 2 2p 6 3s 2 3p 1 | |
| 14 | Si | silicon | 1s 2 2s 2 2p 6 3s 2 3p 2 | |
| 15 | P | phosphorus | 1s 2 2s 2 2p 6 3s 2 3p 3 | |
| 16 | S | sulfur | 1s 2 2s 2 2p 6 3s 2 3p 4 | |
| 17 | Cl | chlorine | 1s 2 2s 2 2p 6 3s 2 3p 5 | |
| 18 | Ar | argon | 1s 2 2s 2 2p 6 3s 2 3p 6 | |
| IV | 19 | K | potassium | 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1 |
| 20 | Ca | calcium | 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 | |
| 21 | sc | scandium | 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 1 | |
| 22 | Ti | titanium | 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 2 | |
| 23 | V | vanadium | 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 3 | |
| 24 | Cr | chromium | 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1 3d 5 s on the d sublevel | |
| 25 | Mn | manganese | 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 5 | |
| 26 | Fe | iron | 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 | |
| 27 | co | cobalt | 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 7 | |
| 28 | Ni | nickel | 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 8 | |
| 29 | Cu | copper | 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1 3d 10 s on the d sublevel | |
| 30 | Zn | zinc | 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 | |
| 31 | Ga | gallium | 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 1 | |
| 32 | Ge | germanium | 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 2 | |
| 33 | As | arsenic | 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 3 | |
| 34 | Se | selenium | 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 4 | |
| 35 | Br | bromine | 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5 | |
| 36 | kr | krypton | 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 |
As already mentioned, in their ground state, electrons in atomic orbitals are arranged according to the principle of least energy. Nevertheless, in the presence of empty p-orbitals in the ground state of an atom, often, when excess energy is imparted to it, the atom can be transferred to the so-called excited state. So, for example, a boron atom in its ground state has an electronic configuration and an energy diagram of the following form:
5 B = 1s 2 2s 2 2p 1
And in the excited state (*), i.e. when imparting some energy to the boron atom, its electronic configuration and energy diagram will look like this:
5 B* = 1s 2 2s 1 2p 2
Depending on which sublevel in the atom is filled last, chemical elements are divided into s, p, d or f.
Finding s, p, d and f-elements in the table D.I. Mendeleev:
- s-elements have the last s-sublevel to be filled. These elements include elements of the main (on the left in the table cell) subgroups of groups I and II.
- For p-elements, the p-sublevel is filled. The p-elements include the last six elements of each period, except for the first and seventh, as well as elements of the main subgroups of III-VIII groups.
- d-elements are located between s- and p-elements in large periods.
- The f-elements are called lanthanides and actinides. They are placed at the bottom of the table by D.I. Mendeleev.
Let us mentally take an atom of any chemical element. What states are the electrons in? It is clear from the previous paragraph that for each electron it is necessary to know the values of four quantum numbers that characterize its state. But we don't yet know how many electrons are in each state. Which conditions are more and which are less likely? These questions are answered by two important principle(law). The first of them was discovered in 1925 by the Swiss physicist V. Pauli (1900-1958) and named after him - the Pauli principle.
All electrons in an atom are in different states, i.e. are characterized by different sets of four quantum numbers.
In this case, the concept of "principle" denotes one of the fundamental laws of nature, which makes the atom what it is - a microparticle of matter with an individual electronic structure for each chemical element. The role of the Pauli principle in nature becomes clearer if we imagine that it does not work. Then the electronic environment of the atomic nucleus loses structural certainty. All electrons roll into some one most favorable state.
It should be noted that this law is valid for all fermions.
A corollary follows from the Pauli principle, which determines the capacity of the orbital, i.e. the number of electrons that can form a single electron cloud. By choosing any of the orbitals, we fix the first three quantum numbers. For example, for an orbital 2 r 2:p = 2, /= 1, mj= 0. But you can also change the spin quantum number m s Two sets of quantum numbers are obtained:
Therefore, an orbital can hold no more than two electrons, and atoms can have one- and two-electron clouds.
Two electrons in the same orbital are called an electron pair.
Knowing the capacity of the orbital, it is easy to understand that the capacity of the energy sublevel is equal to twice the number of orbitals (Table 5.1).
Table 5.1
Structure of sublevels in atoms
A set of electrons of one energy sublevel is called a subshell of an atom.
The capacity of the energy level is the sum of the capacity of the sublevels (Table 5.2). In the first column of the table, in addition to the values of the main quantum number, there are letter designations for the electron shells of the atom.
Table 5.2
The structure of energy levels in atoms
A set of electrons of the same energy level is called the shell of an atom.
The real filling ("settlement") of orbitals, sublevels and levels with electrons determines the second principle - the principle of least energy.
The ground (stable) state of an atom corresponds to the minimum total energy of electrons.
The states of an atom with increased energy are called excited. An atom in an excited state is unstable in the sense that in a very short time (~10 -8 s) it passes into the ground state, radiating energy quanta.
Any physical system is the more stable, the lower its potential energy. Therefore, we invariably observe that a thrown body hits the ground or rolls down a hill, a bent spring straightens, and so on. Also, the electron shells of atoms are in a stable state if the total energy of the electrons is minimal. We already know the set of possible energy states of an atom (see Fig. 5.7). Let us consider how the corresponding sublevels and levels are populated by electrons. In this case, the Pauli principle is strictly observed, which has priority over the principle of least energy and is not violated. We will depict the electronic structure of atoms using energy diagrams and electronic formulas. The energy diagram is a part of the general sequence of sublevels (see Fig. 5.7), containing populated sublevels. The electronic formula lists the occupied sublevels in ascending order of energy, with superscripts indicating the number of electrons. The first two elements of the periodic system can be represented by diagrams I and II. The diagram shows that the position of the 1n* level in the helium atom is lower than in the hydrogen atom, since helium has a larger nuclear charge and electrons are more strongly attracted to the nucleus. The capacity of the first energy level in the helium atom is exhausted.

In the elements following helium, the second energy level is populated. Consider the energy diagrams of the three nearest elements - lithium, beryllium and boron (diagrams III, IV and V).

In lithium and beryllium, the sublevel is populated 2s. The fifth electron of the boron atom begins to populate sublevel 2 R according to the Pauli principle. At carbon and nitrogen atoms, the population of this sublevel continues (diagrams VI and VII).

In the structure of these elements, another important regularity in the formation of electron shells is manifested - Hund's rule (1927).
The basic) 7 state of the atom corresponds to the population of electrons of the maximum number of energetically equivalent orbitals. In this case, the electrons have the same values of spin quantum numbers (all +1/2 or all -1/2).
When considering the energy diagram of an atom, it seems that the transfer of an electron between identical orbitals 2 R does not change its energy. In fact, when electrons move in different orbitals, the repulsion between them decreases, due to which the potential energy still decreases. Electrons occupying single orbitals are called unpaired. Further, when studying the nature of chemical bonds, we will see that the valence of atoms is determined by the number of unpaired electrons. Nitrogen has three unpaired electrons and is indeed trivalent. Suffice it to recall the formula for ammonia NH 3. Carbon, according to the diagram, is divalent. However, upon absorption of a relatively small energy, one electron is transferred from sublevel 25 to sublevel 2r. Carbon goes into an excited state with the electronic formula s 2 2s ( 2p s . In this state, it has four unpaired electrons. A free atom can only be in an excited state for a very short time. But, being in the composition of the molecule, the atom receives additional electrons to populate the orbitals. After that, the possibility of transition to the ground state is excluded, and the carbon atom remains tetravalent. In fact, the energy spent on excitation of an electron is compensated by the energy of formation of additional chemical bonds.
Population of 2p orbitals by second electrons occurs in oxygen, fluorine, and neon (diagrams VIII, IX, X). In this case, the number of remaining unpaired electrons and, accordingly, the valency of atoms decreases successively. This corresponds to elementary knowledge about the properties of oxygen, fluorine and neon: oxygen is bivalent, fluorine is monovalent, and neon does not form chemical bonds, i.e. its valency is zero.

We have seen that the elements from lithium to neon have a second energy level populated by electrons, and that is why they are
- 2nd period of the periodic table. At the sodium following neon, the population of the third energy level begins, and then
- 3rd period as sublevels 35 and 3 are populated R. Energy diagrams and electronic formulas of elements from sodium to argon can be represented in an abbreviated form, designating the repeating set of neon electrons in them as. The meaning of the abbreviated electronic formula is that only the valence electrons of the atom are indicated in it. The rest of the electrons that make up electron core of an atom, are of secondary importance for chemistry. As an example, let's write abbreviated formulas and diagrams for sodium, silicon and argon (diagrams XI, XII and XIII).

The number of chemical elements in the 2nd and 3rd periods is determined by the total capacity of the 5- and /^-sublevels, which is eight electrons. Thus, the presence of exactly eight groups in the periodic table receives a physical explanation. The reason for the observed similarity of chemical elements in groups also becomes clear. Comparing the energy diagrams of elements of the same group - lithium and sodium, carbon and silicon, etc. - we notice that they are characterized by the same population of the external energy level. From this follows, first of all, the same valence of atoms, which is the reason for the similarity of chemical properties. But the electronic structures of atoms, taken as a whole, are different. From period to period, the number of electron shells increases, which entails an increase in the atomic radii. Therefore, as already noted, along with the similarity, there is also a certain direction in the change in properties.
From the electronic formulas and energy diagrams of atoms, it is obvious that in groups IA and PA, electrons fill the outer 5-sublevel, and in groups I HA-V111A, the outer p-sublevel. This provides a basis for classifying chemical elements into blocks. The first two groups are considered as block of s-elements, and groups from ША to VIIIА - as block of p-elements.
In the periodic table there are more groups with the same numbers, but with the addition of the symbol "B". How is the existence of these groups explained? From fig. 5.6 it is obvious that the sublevel 3d energy is between sublevels 45 and 4 R. In the periodic table, the 4th period, like the previous ones, begins with two 5-elements - potassium ([Ar] 45 l) and calcium (fAr] 4l 2). After calcium, the settlement of a non-sublevel begins R, as in the 2nd and 3rd periods, and the sublevel 3d, which has a capacity of 10 electrons. Electrons at the ^-sublevel appear one after another in scandium and the elements following it, including zinc. They are included in block of d-elements. The numbering of groups of d-elements is based on the fact that in groups III to VIII there is the same number of electrons in the two upper sublevels of both p-elements (5- and p-sublevels) and d-elements (5- and d- sublevels). Groups IB and PV are numbered according to the population of the outer 5-sublevel, like 5-elements.
The fourth period is completed by p-elements following zinc. The filled Zr/-nolevel in them is energetically stabilized and becomes lower than the sublevel As. This is explained by the different course of lowering the energy of the orbitals of the 45- and 3^/-sublevels as the charge of the atomic nucleus increases (Fig. 5.9).

Rice. 5.9.
Example 5.1. Write the abbreviated electronic formulas for iron and krypton.
Decision. For both iron and krypton, the nearest antecedent noble gas is argon (Z = 18). Iron (Z = 26) has eight electrons left to fill the upper 45 and 36/ sublevels. We write the formula 45 2 3rf 6 . Krypton (Z = 36) has 10 more electrons added, which completely populate the sublevels 3d and Ar. Filled 3d-set the sublevel in the formula up to the 45th sublevel: [Ar]3 10 45 2 4/? 6.
The fifth period of the periodic table is similar in structure to the fourth. Both of them contain 18 chemical elements. In the 5th period, rubidium and strontium belong to the 5-block of elements, 10 elements from yttrium to cadmium belong to the d-block and the remaining six elements from indium to xenon belong to R- block.
This is followed by the longest 6th and 7th periods containing but 32 elements. In the 6th period, a family of 14 chemical elements is added - from lanthanum to ytterbium, called lanthanides, and in the 7th - a similar family actinides - from actinium to nobelium. In their atoms, the 4/- and 5/-sublevels are filled with electrons, respectively. The lanthanides and actinides make up the block of /-elements. Due to the special characteristics of the orbitals of the / sublevels, all lanthanides and all actinides exhibit a great similarity in chemical properties.
Example 5.2. What explains why families of /-elements contain 14 chemical elements each?
Decision. In accordance with the formula 2/+1 sublevel f(1=3) consists of seven orbitals. Therefore, its capacity is 14 electrons, and the gradual filling of the /-sublevel occurs in 14 chemical elements.
Thus, a brief review of the electronic structure of atoms in general terms reveals the physical basis for the periodicity of changes in the properties of chemical elements and, consequently, the periodic law of D. I. Mendeleev. Briefly, we can say that the periodic law is a consequence of the Pauli principle and the principle of least energy.
The composition of the atom.
An atom is made up of atomic nucleus and electron shell.
The nucleus of an atom is made up of protons ( p+) and neutrons ( n 0). Most hydrogen atoms have a single proton nucleus.
Number of protons N(p+) is equal to the nuclear charge ( Z) and the ordinal number of the element in the natural series of elements (and in the periodic system of elements).
N(p +) = Z
The sum of the number of neutrons N(n 0), denoted simply by the letter N, and the number of protons Z called mass number and is marked with the letter BUT.
A = Z + N
The electron shell of an atom consists of electrons moving around the nucleus ( e -).
Number of electrons N(e-) in the electron shell of a neutral atom is equal to the number of protons Z at its core.
The mass of a proton is approximately equal to the mass of a neutron and 1840 times the mass of an electron, so the mass of an atom is practically equal to the mass of the nucleus.
The shape of an atom is spherical. The radius of the nucleus is about 100,000 times smaller than the radius of the atom.
Chemical element- type of atoms (set of atoms) with the same nuclear charge (with the same number of protons in the nucleus).
Isotope- a set of atoms of one element with the same number of neutrons in the nucleus (or a type of atoms with the same number of protons and the same number of neutrons in the nucleus).
Different isotopes differ from each other in the number of neutrons in the nuclei of their atoms.
Designation of a single atom or isotope: (E - element symbol), for example: .
The structure of the electron shell of the atom
atomic orbital is the state of an electron in an atom. Orbital symbol - . Each orbital corresponds to an electron cloud.
The orbitals of real atoms in the ground (unexcited) state are of four types: s, p, d and f.
electronic cloud- the part of space in which an electron can be found with a probability of 90 (or more) percent.
Note: sometimes the concepts of "atomic orbital" and "electron cloud" are not distinguished, calling both of them "atomic orbital".
The electron shell of an atom is layered. Electronic layer formed by electron clouds of the same size. Orbitals of one layer form electronic ("energy") level, their energies are the same for the hydrogen atom, but different for other atoms.
Orbitals of the same level are grouped into electronic (energy) sublevels:
s- sublevel (consists of one s-orbitals), symbol - .
p sublevel (consists of three p
d sublevel (consists of five d-orbitals), symbol - .
f sublevel (consists of seven f-orbitals), symbol - .
The energies of the orbitals of the same sublevel are the same.
When designating sublevels, the number of the layer (electronic level) is added to the sublevel symbol, for example: 2 s, 3p, 5d means s- sublevel of the second level, p- sublevel of the third level, d- sublevel of the fifth level.
The total number of sublevels in one level is equal to the level number n. The total number of orbitals in one level is n 2. Accordingly, the total number of clouds in one layer is also n 2 .
Designations: - free orbital (without electrons), - orbital with an unpaired electron, - orbital with an electron pair (with two electrons).
The order in which electrons fill the orbitals of an atom is determined by three laws of nature (formulations are given in a simplified way):
1. The principle of least energy - electrons fill the orbitals in order of increasing energy of the orbitals.
2. Pauli's principle - there cannot be more than two electrons in one orbital.
3. Hund's rule - within the sublevel, electrons first fill free orbitals (one at a time), and only after that they form electron pairs.
The total number of electrons in the electronic level (or in the electronic layer) is 2 n 2 .
The distribution of sublevels by energy is expressed next (in order of increasing energy):
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p ...
Visually, this sequence is expressed by the energy diagram:
The distribution of electrons of an atom by levels, sublevels and orbitals (the electronic configuration of an atom) can be depicted as an electronic formula, an energy diagram, or, more simply, as a diagram of electronic layers ("electronic diagram").
Examples of the electronic structure of atoms:
Valence electrons- electrons of an atom that can take part in the formation of chemical bonds. For any atom, these are all the outer electrons plus those pre-outer electrons whose energy is greater than that of the outer ones. For example: Ca atom has 4 outer electrons s 2, they are also valence; the Fe atom has external electrons - 4 s 2 but he has 3 d 6, hence the iron atom has 8 valence electrons. The valence electronic formula of the calcium atom is 4 s 2, and iron atoms - 4 s 2 3d 6 .
Periodic system of chemical elements of D. I. Mendeleev
(natural system of chemical elements)
Periodic law of chemical elements(modern formulation): the properties of chemical elements, as well as simple and complex substances formed by them, are in a periodic dependence on the value of the charge from atomic nuclei.
Periodic system- graphical expression of the periodic law.
Natural range of chemical elements- a number of chemical elements, arranged according to the increase in the number of protons in the nuclei of their atoms, or, what is the same, according to the increase in the charges of the nuclei of these atoms. The serial number of an element in this series is equal to the number of protons in the nucleus of any atom of this element.
The table of chemical elements is constructed by "cutting" the natural series of chemical elements into periods(horizontal rows of the table) and groupings (vertical columns of the table) of elements with a similar electronic structure of atoms.
Depending on how elements are combined into groups, a table can be long period(elements with the same number and type of valence electrons are collected in groups) and short-term(elements with the same number of valence electrons are collected in groups).
The groups of the short period table are divided into subgroups ( main and side effects), coinciding with the groups of the long-period table.
All atoms of elements of the same period have the same number of electron layers, equal to the number of the period.
The number of elements in the periods: 2, 8, 8, 18, 18, 32, 32. Most of the elements of the eighth period were obtained artificially, the last elements of this period have not yet been synthesized. All periods except the first start with an alkali metal forming element (Li, Na, K, etc.) and end with a noble gas forming element (He, Ne, Ar, Kr, etc.).
In the short period table - eight groups, each of which is divided into two subgroups (main and secondary), in the long period table - sixteen groups, which are numbered in Roman numerals with the letters A or B, for example: IA, IIIB, VIA, VIIB. Group IA of the long period table corresponds to the main subgroup of the first group of the short period table; group VIIB - secondary subgroup of the seventh group: the rest - similarly.
The characteristics of chemical elements naturally change in groups and periods.
In periods (with increasing serial number)
- the nuclear charge increases
- the number of outer electrons increases,
- the radius of the atoms decreases,
- the bond strength of electrons with the nucleus increases (ionization energy),
- electronegativity increases.
- the oxidizing properties of simple substances are enhanced ("non-metallicity"),
- the reducing properties of simple substances ("metallicity") weaken,
- weakens the basic character of hydroxides and the corresponding oxides,
- the acidic character of hydroxides and corresponding oxides increases.
In groups (with increasing serial number)
- the nuclear charge increases
- the radius of atoms increases (only in A-groups),
- the strength of the bond between electrons and the nucleus decreases (ionization energy; only in A-groups),
- electronegativity decreases (only in A-groups),
- weaken the oxidizing properties of simple substances ("non-metallicity"; only in A-groups),
- the reducing properties of simple substances are enhanced ("metallicity"; only in A-groups),
- the basic character of hydroxides and the corresponding oxides increases (only in A-groups),
- the acidic nature of hydroxides and the corresponding oxides weakens (only in A-groups),
- the stability of hydrogen compounds decreases (their reducing activity increases; only in A-groups).
Tasks and tests on the topic "Topic 9. "The structure of the atom. Periodic law and periodic system of chemical elements of D. I. Mendeleev (PSCE)"."
- Periodic Law - Periodic law and structure of atoms Grade 8–9
You should know: the laws of filling orbitals with electrons (principle of least energy, Pauli's principle, Hund's rule), the structure of the periodic system of elements.You should be able to: determine the composition of an atom by the position of an element in the periodic system, and, conversely, find an element in the periodic system, knowing its composition; depict the structure diagram, the electronic configuration of an atom, ion, and, conversely, determine the position of a chemical element in the PSCE from the diagram and electronic configuration; characterize the element and the substances it forms according to its position in the PSCE; determine changes in the radius of atoms, the properties of chemical elements and the substances they form within one period and one main subgroup of the periodic system.
Example 1 Determine the number of orbitals in the third electronic level. What are these orbitals?
To determine the number of orbitals, we use the formula N orbitals = n 2 , where n- level number. N orbitals = 3 2 = 9. One 3 s-, three 3 p- and five 3 d-orbitals.Example 2 Determine the atom of which element has the electronic formula 1 s 2 2s 2 2p 6 3s 2 3p 1 .
In order to determine which element it is, you need to find out its serial number, which is equal to the total number of electrons in the atom. In this case: 2 + 2 + 6 + 2 + 1 = 13. This is aluminum.After making sure that everything you need is learned, proceed to the tasks. We wish you success.
Recommended literature:- O. S. Gabrielyan and others. Chemistry, 11th grade. M., Bustard, 2002;
- G. E. Rudzitis, F. G. Feldman. Chemistry 11 cells. M., Education, 2001.
The structure of the electron shells of atoms of the elements of the first four periods: $s-$, $p-$ and $d-$elements. The electronic configuration of the atom. Ground and excited states of atoms
The concept of an atom arose in the ancient world to designate the particles of matter. In Greek, atom means "indivisible".
Electrons
The Irish physicist Stoney, on the basis of experiments, came to the conclusion that electricity is carried by the smallest particles that exist in the atoms of all chemical elements. In $1891$, Stoney proposed to call these particles electrons, which in Greek means "amber".
A few years after the electron got its name, English physicist Joseph Thomson and French physicist Jean Perrin proved that electrons carry a negative charge. This is the smallest negative charge, which in chemistry is taken as the unit $(–1)$. Thomson even managed to determine the speed of the electron (it is equal to the speed of light - $300,000$ km/s) and the mass of the electron (it is $1836$ times less than the mass of the hydrogen atom).
Thomson and Perrin connected the poles of a current source with two metal plates - a cathode and an anode, soldered into a glass tube, from which air was evacuated. When a voltage of about 10 thousand volts was applied to the electrode plates, a luminous discharge flashed in the tube, and particles flew from the cathode (negative pole) to the anode (positive pole), which scientists first called cathode rays, and then found out that it was a stream of electrons. Electrons, hitting special substances applied, for example, to a TV screen, cause a glow.
The conclusion was made: electrons escape from the atoms of the material from which the cathode is made.
Free electrons or their flux can also be obtained in other ways, for example, by heating a metal wire or by falling light on metals formed by elements of the main subgroup of group I of the periodic table (for example, cesium).
The state of electrons in an atom
The state of an electron in an atom is understood as a set of information about energy specific electron in space in which it is located. We already know that an electron in an atom does not have a trajectory of motion, i.e. can only talk about probabilities finding it in the space around the nucleus. It can be located in any part of this space surrounding the nucleus, and the totality of its various positions is considered as an electron cloud with a certain negative charge density. Figuratively, this can be imagined as follows: if it were possible to photograph the position of an electron in an atom in hundredths or millionths of a second, as in a photo finish, then the electron in such photographs would be represented as a point. Overlaying countless such photographs would result in a picture of an electron cloud with the highest density where there are most of these points.
The figure shows a "cut" of such an electron density in a hydrogen atom passing through the nucleus, and a sphere is bounded by a dashed line, inside which the probability of finding an electron is $90%$. The contour closest to the nucleus covers the region of space in which the probability of finding an electron is $10%$, the probability of finding an electron inside the second contour from the nucleus is $20%$, inside the third one - $≈30%$, etc. There is some uncertainty in the state of the electron. To characterize this special state, the German physicist W. Heisenberg introduced the concept of uncertainty principle, i.e. showed that it is impossible to determine simultaneously and exactly the energy and location of the electron. The more accurately the energy of an electron is determined, the more uncertain its position, and vice versa, having determined the position, it is impossible to determine the energy of the electron. The electron detection probability region has no clear boundaries. However, it is possible to single out the space where the probability of finding an electron is maximum.
The space around the atomic nucleus, in which the electron is most likely to be found, is called the orbital.

It contains approximately $90%$ of the electron cloud, which means that about $90%$ of the time the electron is in this part of space. According to the form, $4$ of currently known types of orbitals are distinguished, which are denoted by the Latin letters $s, p, d$ and $f$. A graphic representation of some forms of electronic orbitals is shown in the figure.

The most important characteristic of the motion of an electron in a certain orbit is the energy of its connection with the nucleus. Electrons with similar energy values form a single electronic layer, or energy level. Energy levels are numbered starting from the nucleus: $1, 2, 3, 4, 5, 6$ and $7$.
An integer $n$ denoting the number of the energy level is called the principal quantum number.
It characterizes the energy of electrons occupying a given energy level. The electrons of the first energy level, closest to the nucleus, have the lowest energy. Compared with the electrons of the first level, the electrons of the next levels are characterized by a large amount of energy. Consequently, the electrons of the outer level are the least strongly bound to the nucleus of the atom.
The number of energy levels (electronic layers) in an atom is equal to the number of the period in the system of D. I. Mendeleev, to which the chemical element belongs: the atoms of the elements of the first period have one energy level; the second period - two; seventh period - seven.
The largest number of electrons in the energy level is determined by the formula:
where $N$ is the maximum number of electrons; $n$ is the level number, or the main quantum number. Consequently: the first energy level closest to the nucleus can contain no more than two electrons; on the second - no more than $8$; on the third - no more than $18$; on the fourth - no more than $32$. And how, in turn, are the energy levels (electronic layers) arranged?
Starting from the second energy level $(n = 2)$, each of the levels is subdivided into sublevels (sublayers), which differ somewhat from each other in the binding energy with the nucleus.
The number of sublevels is equal to the value of the main quantum number: the first energy level has one sub level; the second - two; third - three; the fourth is four. Sublevels, in turn, are formed by orbitals.
Each value of $n$ corresponds to the number of orbitals equal to $n^2$. According to the data presented in the table, it is possible to trace the relationship between the principal quantum number $n$ and the number of sublevels, the type and number of orbitals, and the maximum number of electrons per sublevel and level.
Principal quantum number, types and number of orbitals, maximum number of electrons at sublevels and levels.
| Energy level $(n)$ | Number of sublevels equal to $n$ | Orbital type | Number of orbitals | Maximum number of electrons | ||
| in sublevel | in level equal to $n^2$ | in sublevel | at a level equal to $n^2$ | |||
| $K(n=1)$ | $1$ | $1s$ | $1$ | $1$ | $2$ | $2$ |
| $L(n=2)$ | $2$ | $2s$ | $1$ | $4$ | $2$ | $8$ |
| $2p$ | $3$ | $6$ | ||||
| $M(n=3)$ | $3$ | $3s$ | $1$ | $9$ | $2$ | $18$ |
| $3p$ | $3$ | $6$ | ||||
| $3d$ | $5$ | $10$ | ||||
| $N(n=4)$ | $4$ | $4s$ | $1$ | $16$ | $2$ | $32$ |
| $4p$ | $3$ | $6$ | ||||
| $4d$ | $5$ | $10$ | ||||
| $4f$ | $7$ | $14$ | ||||
It is customary to designate sublevels in Latin letters, as well as the shape of the orbitals of which they consist: $s, p, d, f$. So:
- $s$-sublevel - the first sublevel of each energy level closest to the atomic nucleus, consists of one $s$-orbital;
- $p$-sublevel - the second sublevel of each, except for the first, energy level, consists of three $p$-orbitals;
- $d$-sublevel - the third sublevel of each, starting from the third energy level, consists of five $d$-orbitals;
- The $f$-sublevel of each, starting from the fourth energy level, consists of seven $f$-orbitals.
atom nucleus
But not only electrons are part of atoms. Physicist Henri Becquerel discovered that a natural mineral containing uranium salt also emits unknown radiation, illuminating photographic films that are closed from light. This phenomenon has been called radioactivity.
There are three types of radioactive rays:
- $α$-rays, which consist of $α$-particles having a charge $2$ times greater than the charge of an electron, but with a positive sign, and a mass $4$ times greater than the mass of a hydrogen atom;
- $β$-rays are a stream of electrons;
- $γ$-rays are electromagnetic waves with a negligible mass that do not carry an electric charge.
Consequently, the atom has a complex structure - it consists of a positively charged nucleus and electrons.
How is the atom arranged?
In 1910 in Cambridge, near London, Ernest Rutherford with his students and colleagues studied the scattering of $α$ particles passing through thin gold foil and falling on a screen. Alpha particles usually deviated from the original direction by only one degree, confirming, it would seem, the uniformity and uniformity of the properties of gold atoms. And suddenly the researchers noticed that some $α$-particles abruptly changed the direction of their path, as if running into some kind of obstacle.
By placing the screen in front of the foil, Rutherford was able to detect even those rare cases when $α$-particles, reflected from gold atoms, flew in the opposite direction.
Calculations showed that the observed phenomena could occur if the entire mass of the atom and all its positive charge were concentrated in a tiny central nucleus. The radius of the nucleus, as it turned out, is 100,000 times smaller than the radius of the entire atom, that area in which there are electrons that have a negative charge. If we apply a figurative comparison, then the entire volume of the atom can be likened to the Luzhniki stadium, and the nucleus can be likened to a soccer ball located in the center of the field.
An atom of any chemical element is comparable to a tiny solar system. Therefore, such a model of the atom, proposed by Rutherford, is called planetary.
Protons and neutrons
It turns out that the tiny atomic nucleus, in which the entire mass of the atom is concentrated, consists of particles of two types - protons and neutrons.
Protons have a charge equal to the charge of electrons, but opposite in sign $(+1)$, and a mass equal to the mass of a hydrogen atom (it is accepted in chemistry as a unit). Protons are denoted by $↙(1)↖(1)p$ (or $р+$). Neutrons do not carry a charge, they are neutral and have a mass equal to the mass of a proton, i.e. $1$. Neutrons are denoted by $↙(0)↖(1)n$ (or $n^0$).
Protons and neutrons are collectively called nucleons(from lat. nucleus- core).
The sum of the number of protons and neutrons in an atom is called mass number. For example, the mass number of an aluminum atom:

Since the mass of the electron, which is negligible, can be neglected, it is obvious that the entire mass of the atom is concentrated in the nucleus. Electrons are denoted as follows: $e↖(-)$.
Since the atom is electrically neutral, it is also obvious that that the number of protons and electrons in an atom is the same. It is equal to the atomic number of the chemical element assigned to it in the Periodic Table. For example, the nucleus of an iron atom contains $26$ protons, and $26$ electrons revolve around the nucleus. And how to determine the number of neutrons?
As you know, the mass of an atom is the sum of the mass of protons and neutrons. Knowing the ordinal number of the element $(Z)$, i.e. the number of protons, and the mass number $(A)$, equal to the sum of the numbers of protons and neutrons, you can find the number of neutrons $(N)$ using the formula:
For example, the number of neutrons in an iron atom is:
$56 – 26 = 30$.
The table shows the main characteristics of elementary particles.
Basic characteristics of elementary particles.
isotopes
Varieties of atoms of the same element that have the same nuclear charge but different mass numbers are called isotopes.
Word isotope consists of two Greek words: isos- the same and topos- place, means "occupying one place" (cell) in the Periodic system of elements.
Chemical elements found in nature are a mixture of isotopes. Thus, carbon has three isotopes with a mass of $12, 13, 14$; oxygen - three isotopes with a mass of $16, 17, 18$, etc.
Usually given in the Periodic system, the relative atomic mass of a chemical element is the average value of the atomic masses of a natural mixture of isotopes of a given element, taking into account their relative abundance in nature, therefore, the values of atomic masses are quite often fractional. For example, natural chlorine atoms are a mixture of two isotopes - $35$ (there are $75%$ in nature) and $37$ (there are $25%$); therefore, the relative atomic mass of chlorine is $35.5$. Isotopes of chlorine are written as follows:
$↖(35)↙(17)(Cl)$ and $↖(37)↙(17)(Cl)$
The chemical properties of chlorine isotopes are exactly the same, as are the isotopes of most chemical elements, such as potassium, argon:
$↖(39)↙(19)(K)$ and $↖(40)↙(19)(K)$, $↖(39)↙(18)(Ar)$ and $↖(40)↙(18 )(Ar)$
However, hydrogen isotopes differ greatly in properties due to the dramatic fold increase in their relative atomic mass; they were even given individual names and chemical signs: protium - $↖(1)↙(1)(H)$; deuterium - $↖(2)↙(1)(H)$, or $↖(2)↙(1)(D)$; tritium - $↖(3)↙(1)(H)$, or $↖(3)↙(1)(T)$.

Now it is possible to give a modern, more rigorous and scientific definition of a chemical element.
A chemical element is a collection of atoms with the same nuclear charge.
The structure of the electron shells of atoms of the elements of the first four periods
Consider the mapping of the electronic configurations of the atoms of the elements by the periods of the system of D. I. Mendeleev.
Elements of the first period.
Schemes of the electronic structure of atoms show the distribution of electrons over electronic layers (energy levels).

The electronic formulas of atoms show the distribution of electrons over energy levels and sublevels.
Graphic electronic formulas of atoms show the distribution of electrons not only in levels and sublevels, but also in orbitals.

In a helium atom, the first electron layer is complete - it has $2$ electrons.
Hydrogen and helium are $s$-elements, these atoms have $s$-orbitals filled with electrons.
Elements of the second period.
For all elements of the second period, the first electron layer is filled, and the electrons fill the $s-$ and $p$ orbitals of the second electron layer in accordance with the principle of least energy (first $s$, and then $p$) and the rules of Pauli and Hund.
In the neon atom, the second electron layer is complete - it has $8$ electrons.
Elements of the third period.
For atoms of elements of the third period, the first and second electron layers are completed, so the third electron layer is filled, in which electrons can occupy 3s-, 3p- and 3d-sublevels.
The structure of the electron shells of atoms of the elements of the third period.
A $3.5$-electron orbital is completed at the magnesium atom. $Na$ and $Mg$ are $s$-elements.
For aluminum and subsequent elements, the $3d$ sublevel is filled with electrons.
| $↙(18)(Ar)$ Argon |  |
$1s^2(2)s^2(2)p^6(3)s^2(3)p^6$ |  |
In an argon atom, the outer layer (the third electron layer) has $8$ electrons. As the outer layer is completed, but in total, in the third electron layer, as you already know, there can be 18 electrons, which means that the elements of the third period have $3d$-orbitals left unfilled.
All elements from $Al$ to $Ar$ - $p$ -elements.
$s-$ and $r$ -elements form main subgroups in the Periodic system.
Elements of the fourth period.
Potassium and calcium atoms have a fourth electron layer, the $4s$-sublevel is filled, because it has less energy than the $3d$-sublevel. To simplify the graphical electronic formulas of the atoms of the elements of the fourth period:
- we denote conditionally the graphic electronic formula of argon as follows: $Ar$;
- we will not depict the sublevels that are not filled for these atoms.
$K, Ca$ - $s$ -elements, included in the main subgroups. For atoms from $Sc$ to $Zn$, the 3d sublevel is filled with electrons. These are $3d$-elements. They are included in side subgroups, their pre-external electron layer is filled, they are referred to transition elements.
Pay attention to the structure of the electron shells of chromium and copper atoms. A "failure" of one electron from the $4s-$ to the $3d$ sublevel occurs in them, which is explained by the greater energy stability of the resulting $3d^5$ and $3d^(10)$ electronic configurations:
$↙(24)(Cr)$ $1s^(2)2s^(2)2p^(6)3s^(2)3p^(6)3d^(4) 4s^(2)…$ 
$↙(29)(Cu)$ $1s^(2)2s^(2)2p^(6)3s^(2)3p^(6)3d^(9)4s^(2)…$ 
| Element symbol, serial number, name | Diagram of the electronic structure | Electronic formula | Graphic electronic formula |
| $↙(19)(K)$ Potassium |  |
$1s^2(2)s^2(2)p^6(3)p^6(4)s^1$ | |
| $↙(20)(C)$ Calcium |  |
$1s^2(2)s^2(2)p^6(3)p^6(4)s^2$ | |
| $↙(21)(Sc)$ Scandium |  |
$1s^2(2)s^2(2)p^6(3)p^6(4)s^1(3)d^1$ or $1s^2(2)s^2(2)p ^6(3)p^6(3)d^1(4)s^1$ |  |
| $↙(22)(Ti)$ Titanium |  |
$1s^2(2)s^2(2)p^6(3)p^6(4)s^2(3)d^2$ or $1s^2(2)s^2(2)p ^6(3)p^6(3)d^2(4)s^2$ |  |
| $↙(23)(V)$ Vanadium |  |
$1s^2(2)s^2(2)p^6(3)p^6(4)s^2(3)d^3$ or $1s^2(2)s^2(2)p ^6(3)p^6(3)d^3(4)s^2$ |  |
| $↙(24)(Cr)$ Chrome |  |
$1s^2(2)s^2(2)p^6(3)p^6(4)s^1(3)d^5$ or $1s^2(2)s^2(2)p ^6(3)p^6(3)d^5(4)s^1$ |  |
| $↙(29)(Сu)$ Chromium |  |
$1s^2(2)s^2(2)p^6(3)p^6(4)s^1(3)d^(10)$ or $1s^2(2)s^2(2 )p^6(3)p^6(3)d^(10)(4)s^1$ |  |
| $↙(30)(Zn)$ Zinc |  |
$1s^2(2)s^2(2)p^6(3)p^6(4)s^2(3)d^(10)$ or $1s^2(2)s^2(2 )p^6(3)p^6(3)d^(10)(4)s^2$ |  |
| $↙(31)(Ga)$ Gallium |  |
$1s^2(2)s^2(2)p^6(3)p^6(4)s^2(3)d^(10)4p^(1)$ or $1s^2(2) s^2(2)p^6(3)p^6(3)d^(10)(4)s^(2)4p^(1)$ |  |
| $↙(36)(Kr)$ Krypton |  |
$1s^2(2)s^2(2)p^6(3)p^6(4)s^2(3)d^(10)4p^6$ or $1s^2(2)s^ 2(2)p^6(3)p^6(3)d^(10)(4)s^(2)4p^6$ |  |
In the zinc atom, the third electron layer is complete - all the $3s, 3p$ and $3d$ sublevels are filled in it, in total there are $18$ of electrons on them.
In the elements following zinc, the fourth electron layer, the $4p$-sublevel, continues to be filled. Elements from $Ga$ to $Kr$ - $r$ -elements.
The outer (fourth) layer of a krypton atom is completed, it has $8$ of electrons. But just in the fourth electron layer, as you know, there can be $32$ of electrons; the krypton atom still has $4d-$ and $4f$-sublevels unfilled.
The elements of the fifth period are filling the sublevels in the following order: $5s → 4d → 5р$. And there are also exceptions related to the "failure" of electrons, for $↙(41)Nb$, $↙(42)Mo$, $↙(44)Ru$, $↙(45)Rh$, $↙(46) Pd$, $↙(47)Ag$. $f$ appear in the sixth and seventh periods -elements, i.e. elements whose $4f-$ and $5f$-sublevels of the third outside electronic layer are being filled, respectively.
$4f$ -elements called lanthanides.
$5f$ -elements called actinides.
The order of filling of electronic sublevels in the atoms of elements of the sixth period: $↙(55)Cs$ and $↙(56)Ba$ - $6s$-elements; $↙(57)La ... 6s^(2)5d^(1)$ - $5d$-element; $↙(58)Ce$ – $↙(71)Lu - 4f$-elements; $↙(72)Hf$ – $↙(80)Hg - 5d$-elements; $↙(81)Т1$ – $↙(86)Rn - 6d$-elements. But even here there are elements in which the order of filling of electron orbitals is violated, which, for example, is associated with greater energy stability of half and completely filled $f$-sublevels, i.e. $nf^7$ and $nf^(14)$.
Depending on which sublevel of the atom is filled with electrons last, all elements, as you already understood, are divided into four electronic families, or blocks:
- $s$ -elements; the $s$-sublevel of the outer level of the atom is filled with electrons; $s$-elements include hydrogen, helium and elements of the main subgroups of groups I and II;
- $r$ -elements; the $p$-sublevel of the outer level of the atom is filled with electrons; $p$-elements include elements of the main subgroups of groups III–VIII;
- $d$ -elements; the $d$-sublevel of the preexternal level of the atom is filled with electrons; $d$-elements include elements of secondary subgroups of groups I–VIII, i.e. elements of intercalated decades of large periods located between $s-$ and $p-$elements. They are also called transition elements;
- $f$ -elements;$f-$sublevel of the third level of the atom outside is filled with electrons; these include lanthanides and actinides.

The electronic configuration of the atom. Ground and excited states of atoms
The Swiss physicist W. Pauli in $1925$ established that An atom can have at most two electrons in one orbital. having opposite (antiparallel) spins (translated from English as a spindle), i.e. possessing such properties that can be conditionally imagined as the rotation of an electron around its imaginary axis clockwise or counterclockwise. This principle is called the Pauli principle.
If there is one electron in an orbital, then it is called unpaired, if two, then this paired electrons, i.e. electrons with opposite spins.
The figure shows a diagram of the division of energy levels into sublevels.

$s-$ Orbital, as you already know, has a spherical shape. The hydrogen atom electron $(n = 1)$ is located on this orbital and is unpaired. According to this his electronic formula, or electronic configuration, is written like this: $1s^1$. In electronic formulas, the number of the energy level is indicated by the number in front of the letter $ (1 ...) $, the Latin letter denotes the sublevel (orbital type), and the number that is written to the right of the letter (as an exponent) shows the number of electrons in the sublevel.
For a helium atom He, which has two paired electrons in the same $s-$orbital, this formula is: $1s^2$. The electron shell of the helium atom is complete and very stable. Helium is a noble gas. The second energy level $(n = 2)$ has four orbitals, one $s$ and three $p$. Second-level $s$-orbital electrons ($2s$-orbitals) have a higher energy, because are at a greater distance from the nucleus than the electrons of the $1s$-orbital $(n = 2)$. In general, for each value of $n$, there is one $s-$orbital, but with a corresponding amount of electron energy on it and, therefore, with a corresponding diameter, growing as the value of $n$.$s-$Orbital increases, as you already know , has a spherical shape. The hydrogen atom electron $(n = 1)$ is located on this orbital and is unpaired. Therefore, its electronic formula, or electronic configuration, is written as follows: $1s^1$. In electronic formulas, the number of the energy level is indicated by the number in front of the letter $ (1 ...) $, the Latin letter denotes the sublevel (orbital type), and the number that is written to the right of the letter (as an exponent) shows the number of electrons in the sublevel.
For a helium atom $He$, which has two paired electrons in the same $s-$orbital, this formula is: $1s^2$. The electron shell of the helium atom is complete and very stable. Helium is a noble gas. The second energy level $(n = 2)$ has four orbitals, one $s$ and three $p$. Electrons of $s-$orbitals of the second level ($2s$-orbitals) have a higher energy, because are at a greater distance from the nucleus than the electrons of the $1s$-orbital $(n = 2)$. In general, for each value of $n$ there is one $s-$orbital, but with a corresponding amount of electron energy on it and, therefore, with a corresponding diameter, growing as the value of $n$ increases. 
$r-$ Orbital It has the shape of a dumbbell, or volume eight. All three $p$-orbitals are located in the atom mutually perpendicularly along the spatial coordinates drawn through the nucleus of the atom. It should be emphasized again that each energy level (electronic layer), starting from $n= 2$, has three $p$-orbitals. As the value of $n$ increases, the electrons occupy $p$-orbitals located at large distances from the nucleus and directed along the $x, y, z$ axes.
For elements of the second period $(n = 2)$, first one $s$-orbital is filled, and then three $p$-orbitals; electronic formula $Li: 1s^(2)2s^(1)$. The $2s^1$ electron is weaker bound to the atomic nucleus, so a lithium atom can easily give it away (as you probably remember, this process is called oxidation), turning into a lithium ion $Li^+$.
In the beryllium atom Be, the fourth electron is also placed in the $2s$ orbital: $1s^(2)2s^(2)$. The two outer electrons of the beryllium atom are easily detached - $B^0$ is oxidized into the $Be^(2+)$ cation.
The fifth electron of the boron atom occupies the $2p$-orbital: $1s^(2)2s^(2)2p^(1)$. Next, the $2p$-orbitals of the $C, N, O, F$ atoms are filled, which ends with the neon noble gas: $1s^(2)2s^(2)2p^(6)$.
For elements of the third period, $3s-$ and $3p$-orbitals are filled, respectively. Five $d$-orbitals of the third level remain free:
$↙(11)Na 1s^(2)2s^(2)2p^(6)3s^(1)$,
$↙(17)Cl 1s^(2)2s^(2)2p^(6)3s^(2)3p^(5)$,
$↙(18)Ar 1s^(2)2s^(2)2p^(6)3s^(2)3p^(6)$.
Sometimes, in diagrams depicting the distribution of electrons in atoms, only the number of electrons at each energy level is indicated, i.e. write abbreviated electronic formulas of atoms of chemical elements, in contrast to the above full electronic formulas, for example:
$↙(11)Na 2, 8, 1;$ $↙(17)Cl 2, 8, 7;$ $↙(18)Ar 2, 8, 8$.
For elements of large periods (fourth and fifth), the first two electrons occupy respectively $4s-$ and $5s$-orbitals: $↙(19)K 2, 8, 8, 1;$ $↙(38)Sr 2, 8, 18, 8, 2$. Starting from the third element of each large period, the next ten electrons will go to the previous $3d-$ and $4d-$orbitals, respectively (for elements of secondary subgroups): $↙(23)V 2, 8, 11, 2;$ $↙( 26)Fr 2, 8, 14, 2;$ $↙(40)Zr 2, 8, 18, 10, 2;$ $↙(43)Tc 2, 8, 18, 13, 2$. As a rule, when the previous $d$-sublevel is filled, the outer (respectively $4p-$ and $5p-$) $p-$sublevel will start to be filled: $↙(33)As 2, 8, 18, 5;$ $ ↙(52)Te 2, 8, 18, 18, 6$.
For elements of large periods - the sixth and incomplete seventh - electronic levels and sublevels are filled with electrons, as a rule, as follows: the first two electrons enter the outer $s-$sublevel: $↙(56)Ba 2, 8, 18, 18, 8, 2;$ $↙(87)Fr 2, 8, 18, 32, 18, 8, 1$; the next one electron (for $La$ and $Ca$) to the previous $d$-sublevel: $↙(57)La 2, 8, 18, 18, 9, 2$ and $↙(89)Ac 2, 8, 18, 32, 18, 9, 2$.
Then the next $14$ of electrons will enter the third energy level from the outside, the $4f$ and $5f$ orbitals of the lantonides and actinides, respectively: $↙(64)Gd 2, 8, 18, 25, 9, 2;$ $↙(92 )U 2, 8, 18, 32, 21, 9, 2$.
Then the second energy level from the outside ($d$-sublevel) will begin to build up again for the elements of side subgroups: $↙(73)Ta 2, 8, 18, 32, 11, 2;$ $↙(104)Rf 2, 8, 18 , 32, 32, 10, 2$. And, finally, only after the $d$-sublevel is completely filled with ten electrons, the $p$-sublevel will be filled again: $↙(86)Rn 2, 8, 18, 32, 18, 8$.
Very often, the structure of the electron shells of atoms is depicted using energy or quantum cells - they write down the so-called graphic electronic formulas. For this record, the following notation is used: each quantum cell is denoted by a cell that corresponds to one orbital; each electron is indicated by an arrow corresponding to the direction of the spin. When writing a graphical electronic formula, two rules should be remembered: Pauli principle, according to which a cell (orbital) can have no more than two electrons, but with antiparallel spins, and F. Hund's rule, according to which electrons occupy free cells first one at a time and have the same spin value, and only then pair, but the spins, according to the Pauli principle, will already be oppositely directed.
The purpose of the lesson: To form students' ideas about the structure of the electron shell of an atom using the example of chemical elements of 1-3 periods of the periodic system. To consolidate the concepts of "periodic law" and "periodic system".
Lesson objectives: To learn how to make electronic formulas of atoms, determine elements by their electronic formulas, determine the composition of an atom.
Equipment: Periodic system of chemical elements D.I. Mendeleev, blackboard, multimedia projector, personal computer, layout and presentation “Compilation of electronic formulas for the structure of atoms”.
Lesson type: combined
Methods: verbal, visual.
During the classes
I. Organizational moment.
Greetings. Mark absent. Activation of the class for the assimilation of a new topic.
The teacher pronounces and writes down the topic of the lesson on the board “The structure of the electron shells of the atom”.
II. Explanation of new material
Teacher: At the beginning of the 20th century, it was adopted planetary model of the structure of the atom, proposed by Rutherford, according to which electrons move around a very small positively charged nucleus, like planets around the Sun. ( Presentation. slide 1. Rutherford model).
Therefore, in an atom there are trajectories along which an electron moves. However, further studies showed that there are no trajectories of electrons in the atom. Movement without a trajectory means that we do not know how the electron moves in the atom, but we can determine the region where the electron is most often encountered. It's not an orbit, it's an orbital . Moving around the atom, the electrons together form its electron shell.
Let's find out how electrons move around the nucleus? Randomly or in a certain order? Research Niels Bohr- the founder of modern atomic physics, as well as a number of other scientists, made it possible to conclude that electrons in atoms are arranged in certain layers - shells and in a certain order.
The structure of the electron shells of atoms is important for chemistry, since it is the electrons that determine the chemical properties of substances. The most important characteristic of the motion of an electron in a certain orbit is the energy of its connection with the nucleus. Electrons in an atom differ in a certain energy, and, as experiments show, some are attracted to the nucleus more strongly, others weaker. This is explained by the remoteness of electrons from the nucleus. The closer the electrons to the nucleus, the greater their bond with the nucleus, but the less energy. As the distance from the nucleus of the atom, the force of attraction of the electron to the nucleus decreases, and the energy supply increases. This is how electronic layers in the electron shell of an atom. Electrons with similar energy values form a single electron layer, or energy level. The energy of electrons in an atom and the energy level is determined by the main quantum number n and takes integer values 1, 2, 3, 4, 5, 6 and 7. The larger the value of n, the greater the energy of the electron in the atom. The maximum number of electrons that can be in a particular energy level is determined by the formula:
Where N is the maximum number of electrons per level;
n is the number of the energy level.
It has been established that no more than two electrons are located on the first shell, no more than eight on the second, no more than 18 on the third, and no more than 32 on the fourth. We will not consider the filling of more distant shells. It is known that the outer energy level can contain no more than eight electrons, it is called completed. The electron layers that do not contain the maximum number of electrons are called unfinished.
The number of electrons in the outer energy level of the electron shell of an atom is equal to the group number for the chemical elements of the main subgroups.
As previously mentioned, the electron does not move in an orbit, but in an orbit and has no trajectory.
The space around the nucleus where it is most likely to find a given electron is called the orbital of this electron, or electron cloud.
Orbitals, or sublevels, as they are also called, can have different shapes, and their number corresponds to the level number, but does not exceed four. The first energy level has one sublevel ( s), the second - two ( s,p), the third - three ( s,p,d) etc. Electrons of different sublevels of the same level have a different shape of the electron cloud: spherical (s), dumbbell (p) and more complex configuration (d) and (f). Scientists agreed to call the spherical atomic orbital s-orbital. It is the most stable and is located quite close to the core.
The greater the energy of an electron in an atom, the faster it rotates, the more the region of its stay is extended, and, finally, it turns into a dumbbell-shaped p-orbital:
![]()
An electron cloud of this shape can occupy in an atom three positions along the coordinate axes of space x, y and z. This is easily explained: after all, all electrons are negatively charged, so electron clouds repel each other and tend to stay as far away from each other as possible.
So, p There can be three orbitals. Their energy, of course, is the same, but their location in space is different.
Draw a diagram of the sequential filling of energy levels with electrons

Now we can draw up a diagram of the structure of the electron shells of atoms:
- We determine the total number of electrons on the shell by the ordinal number of the element.
- We determine the number of energy levels in the electron shell. Their number is equal to the number of the period in the table of D. I. Mendeleev, in which the element is located.
- Determine the number of electrons in each energy level.
- Using Arabic numerals to designate the level and designating the orbitals with the letters s and p, and the number of electrons in a given orbital with an Arabic numeral in the upper right above the letter, we depict the structure of atoms with more complete electronic formulas. Scientists agreed to designate each atomic orbital quantum cell- a square on energy diagram:
On the s -sublevel can be one atomic orbital
and on p- their sublevel may already be three -
![]()
(according to the three coordinate axes):
Orbitals d– and f- sublevel in an atom may already be five and seven respectively:


The nucleus of a hydrogen atom has a charge of +1, so only one electron moves around its nucleus at a single energy level. Let's write down the electronic configuration of the hydrogen atom
![]()
To establish a connection between the structure of the atom of a chemical element and its properties, we will consider a few more chemical elements.
The next element after hydrogen is helium. The nucleus of a helium atom has a charge of +2, so a helium atom contains two electrons in the first energy level:
![]()
Since the first energy level can contain no more than two electrons, it is considered completed.
Element number 3 - lithium. The lithium nucleus has a charge of +3, therefore, there are three electrons in the lithium atom. Two of them are at the first energy level, and the third electron begins to fill the second energy level. First, the s-orbital of the first level is filled, then the s-orbital of the second level. The electron in the second level is weaker bound to the nucleus than the other two.

For a carbon atom, it is already possible to assume three possible schemes for filling electron shells in accordance with electron-graphic formulas:
An analysis of the atomic spectrum shows that the latter scheme is correct. Using this rule, it is not difficult to draw up a diagram of the electronic structure for the nitrogen atom:

This scheme corresponds to the formula 1s 2 2s 2 2p 3 . Then the pairwise placement of electrons into 2p orbitals begins. Electronic formulas of the remaining atoms of the second period:
The filling of the second energy level of the neon atom ends, and the construction of the second period of the system of elements is completed.
Find the chemical sign of lithium in the periodic system, from lithium to neon Ne, the charge of atomic nuclei naturally increases. The second layer is gradually filled with electrons. With an increase in the number of electrons in the second layer, the metallic properties of the elements gradually weaken and are replaced by non-metallic ones.
The third period, like the second, begins with two elements (Na, Mg), in which the electrons are located on the s-sublevel of the outer electron layer. This is followed by six elements (from Al to Ar), in which the p-sublevel of the outer electron layer is formed. The structure of the outer electronic layer of the corresponding elements of the second and third periods is similar. In other words, with an increase in the charge of the nucleus, the electronic structure of the outer layers of atoms is periodically repeated. If the elements have the same external energy levels, then the properties of these elements are similar. For example, argon and neon contain eight electrons at the outer level, and therefore they are inert, that is, they almost do not enter into chemical reactions. In the free form, argon and neon are gases that have monatomic molecules.
The atoms of lithium, sodium and potassium contain one electron at the outer level and have similar properties, so they are placed in the same group of the periodic system.
III. Findings.
1. The properties of chemical elements, arranged in ascending order of the charge of the nucleus, are periodically repeated, since the structure of the external energy levels of the atoms of the elements is periodically repeated.
2. A smooth change in the properties of chemical elements within one period can be explained by a gradual increase in the number of electrons at the external energy level.
3. The reason for the similarity of the properties of chemical elements belonging to the same family lies in the same structure of the external energy levels of their atoms.
IV. Consolidation of new material.
Task for the class:
1. Draw the structure of the atoms of the following elements:
a) sodium;
b) silicon
2. Compare the structure of nitrogen and phosphorus atoms.
3. According to the distribution of valence electrons, find the element:
a) 1s 2 2s 1
b) 1s 2 2s 2 2p 6 3s 2 3p 6
c) 1s 2 2s 2 2p 6 3s 2 3p 4
d) 1s 2 2s 2 2p 4
e) 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1
4. Using the computer presentation "Compilation of electronic formulas of the structure of atoms" make electronic formulas of atoms a) nitrogen; b) sulfur .
5. Using the layout “Compilation of electronic formulas of the structure of atoms” electronic formulas of atoms: a) magnesium; b) oxygen.
V. Homework: § 8, p. 28-33.
Draw diagrams of the structure of the electron shells of atoms: boron, chlorine, lithium, aluminum.