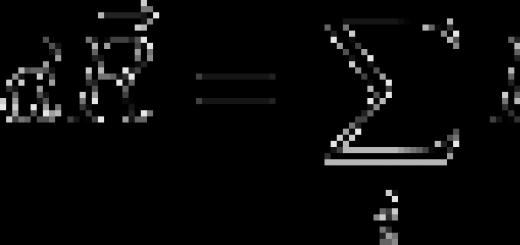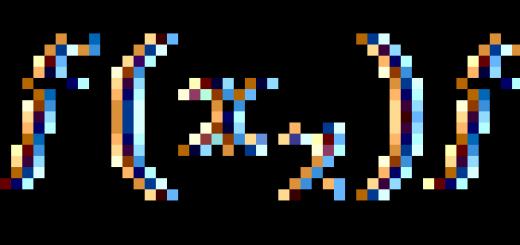constant bar, what is the constant bar equal to
Constant Planck(quantum of action) - the main constant of quantum theory, a coefficient that relates the amount of energy of a quantum of electromagnetic radiation with its frequency, as well as in general the amount of an energy quantum of any linear oscillatory physical system with its frequency. Associates energy and momentum with frequency and spatial frequency, actions with phase. It is a quantum of angular momentum. First mentioned by Planck in his work on thermal radiation, and therefore named after him. The usual designation is Latin. J s erg s. eV s.
Often used value:
J s, erg s, eV s,
called the reduced (sometimes rationalized or reduced) Planck constant or Dirac constant. The use of this notation simplifies many formulas of quantum mechanics, since the traditional Planck constant enters into these formulas in the form divided by a constant.
At the 24th General Conference on Weights and Measures on October 17-21, 2011, a resolution was unanimously adopted, in which, in particular, it was proposed in a future revision of the International System of Units (SI) to redefine the SI units in such a way that the Planck constant was exactly equal to 6.62606X·10−34 J·s, where X replaces one or more significant figures to be determined in the future based on the best CODATA recommendations. In the same resolution, it was proposed to determine in the same way the exact values of the Avogadro constant, the elementary charge, and the Boltzmann constant.
- 1 Physical meaning
- 2 Discovery history
- 2.1 Planck's formula for thermal radiation
- 2.2 Photoelectric effect
- 2.3 Compton effect
- 3 Measurement methods
- 3.1 Using the laws of the photoelectric effect
- 3.2 Analysis of the bremsstrahlung spectrum
- 4 Notes
- 5 Literature
- 6 Links
physical meaning
In quantum mechanics, momentum has the physical meaning of a wave vector, energy - frequencies, and action - wave phases, however, traditionally (historically) mechanical quantities are measured in other units (kg m / s, J, J s) than the corresponding wave (m −1, s −1, dimensionless phase units). Planck's constant plays the role of a conversion factor (always the same) connecting these two systems of units - quantum and traditional:
(momentum) (energy) (action)
If the system of physical units were formed after the advent of quantum mechanics and adapted to simplify the basic theoretical formulas, Planck's constant would probably simply be made equal to one, or, in any case, to a more round number. In theoretical physics, the c system of units is often used to simplify formulas, in which
.Planck's constant also has a simple evaluative role in delimiting the areas of applicability of classical and quantum physics: in comparison with the magnitude of the action or angular momentum values characteristic of the system under consideration, or the products of the characteristic momentum by the characteristic size, or the characteristic energy by the characteristic time, it shows how applicable to a given physical system classical mechanics. Namely, if is the action of the system, and is its angular momentum, then at or the behavior of the system is described with good accuracy by classical mechanics. These estimates are fairly directly related to the Heisenberg uncertainty relations.
Discovery history
Planck's formula for thermal radiation
Main article: Planck formulaPlanck's formula is an expression for the spectral power density of radiation from a black body, which was obtained by Max Planck for the equilibrium radiation density. Planck's formula was obtained after it became clear that the Rayleigh-Jeans formula satisfactorily describes radiation only in the region of long waves. In 1900, Planck proposed a formula with a constant (later called Planck's constant), which agreed well with experimental data. At the same time, Planck believed that this formula is just a successful mathematical trick, but has no physical meaning. That is, Planck did not assume that electromagnetic radiation is emitted in the form of separate portions of energy (quanta), the magnitude of which is related to the radiation frequency by the expression:
The proportionality factor was subsequently called Planck's constant, = 1.054 10−34 J s.
photoelectric effect
Main article: photoelectric effectThe photoelectric effect is the emission of electrons by a substance under the influence of light (and, generally speaking, any electromagnetic radiation). condensed substances (solid and liquid) emit an external and internal photoelectric effect.
The photoelectric effect was explained in 1905 by Albert Einstein (for which he received the Nobel Prize in 1921 thanks to the nomination of the Swedish physicist Oseen) based on Planck's hypothesis about the quantum nature of light. Einstein's work contained an important new hypothesis - if Planck suggested that light is emitted only in quantized portions, then Einstein already believed that light exists only in the form of quantized portions. From the law of conservation of energy, when light is represented in the form of particles (photons), Einstein's formula for the photoelectric effect follows:
where - so-called. work function (the minimum energy required to remove an electron from a substance), is the kinetic energy of an emitted electron, is the frequency of an incident photon with energy, is Planck's constant. From this formula follows the existence of the red border of the photoelectric effect, that is, the existence of the lowest frequency, below which the photon energy is no longer sufficient to “knock out” an electron from the body. The essence of the formula is that the energy of a photon is spent on the ionization of an atom of a substance, that is, on the work necessary to “pull out” an electron, and the remainder is converted into the kinetic energy of an electron.
Compton effect
Main article: Compton effectMeasurement methods
Using the laws of the photoelectric effect
With this method of measuring the Planck constant, Einstein's law for the photoelectric effect is used:
where is the maximum kinetic energy of photoelectrons emitted from the cathode,
The frequency of the incident light, - the so-called. work function of an electron.
The measurement is carried out as follows. First, the cathode of the photocell is irradiated with monochromatic light with a frequency, while a blocking voltage is applied to the photocell, so that the current through the photocell stops. In this case, the following relation takes place, which follows directly from Einstein's law:
where is the electron charge.
Then the same photocell is irradiated with monochromatic light with a frequency and in the same way it is locked with a voltage
Subtracting the second expression term by term from the first one, we obtain
whence it follows
Analysis of the bremsstrahlung spectrum
This method is considered the most accurate of the existing ones. The fact that the frequency spectrum of bremsstrahlung X-rays has a sharp upper limit, called the violet border, is used. Its existence follows from the quantum properties of electromagnetic radiation and the law of conservation of energy. Really,
where is the speed of light,
X-ray wavelength, - electron charge, - accelerating voltage between the electrodes of the X-ray tube.
Then Planck's constant is
Notes
- 1 2 3 4 Fundamental Physical Constants - Complete Listing
- On the possible future revision of the International System of Units, the SI. Resolution 1 of the 24th meeting of the CGPM (2011).
- Agreement to tie kilogram and friends to fundamentals - physics-math - 25 October 2011 - New Scientist
Literature
- John D. Barrow. The Constants of Nature; From Alpha to Omega - The Numbers that Encode the Deepest Secrets of the Universe. - Pantheon Books, 2002. - ISBN 0-37-542221-8.
- Steiner R. History and progress on accurate measurements of the Planck constant // Reports on Progress in Physics. - 2013. - Vol. 76. - P. 016101.
Links
- Yu. K. Zemtsov, Lectures on atomic physics, dimensional analysis
- History of refinement of Planck's constant
- The NIST Reference on Constants, Units and Uncertainty
constant bar, what is the constant bar equal to
Planck's Constant Information About
Sokolnikov Mikhail Leonidovich,
Akhmetov Alexey Lirunovich
Sverdlovsk Regional Non-Governmental Fund
promoting the development of science, culture and art Patron
Russia, Yekaterinburg
Email: [email protected]
Abstract: The relationship between Planck's constant and Wien's law and Kepler's third law is shown. The exact value of Planck's constant for a liquid or solid state of aggregation of a substance, equal to
h \u003d 4 * 10 -34 j * sec.
A formula has been derived that combines four physical constants - the speed of light - c, Wien's constant - v, Planck's constant - h and Boltzmann's constant - k
Keywords: Planck's constant, Wien's constant, Boltzmann's constant, Kepler's third law, quantum mechanics
The Foundation "Maecenas"
Sokolnikov M.L., Akhmetov A.L.
Yekaterinburg, Russian Federation
Email: [email protected]
Abstract: The connection to the Planck constant with Wien's displacement law and Kepler's third law. The exact value of Planck's constant for the liquid or solid state of aggregation of matter equal to
h \u003d 4 * 10 -34 J * s.
The formula that combines four physical constants - the speed of light - c,
Wien "s displacement constant - in, Planck constant - h and the Boltzmann constant - k
Keywords: Planck constant, Wien's displacement constant, the Boltzmann constant, Kepler's third law, quantum mechanics
This physical constant was first stated by the German physicist Max Planck in 1899. In this article, we will try to answer three questions:
1. What is the physical meaning of Planck's constant?
2. How can it be calculated from real experimental data?
3. Is the assertion that energy can be transferred only in certain portions - quanta connected with the Planck constant?
Introduction
Reading modern scientific literature, one involuntarily pays attention to how difficult, and sometimes vaguely, the authors display this topic. Therefore, in my article I will try to explain the situation in simple Russian, without going beyond the level of school formulas. This story began in the second half of the 19th century, when scientists began to study in detail the processes of thermal radiation of bodies. To improve the measurement accuracy in these experiments, special chambers were used, which made it possible to bring the energy absorption coefficient closer to unity. The device of these cameras is described in detail in various sources and I will not dwell on this, I will only note that they can be made from almost any material. It turned out that heat radiation is the radiation of electromagnetic waves in the infrared range, i.e. at frequencies slightly below the visible spectrum. In the course of the experiments, it was found that at any particular body temperature in the IR radiation spectrum of this body, a peak of the maximum intensity of this radiation is observed. As the temperature increased, this peak shifted towards shorter wavelengths; to the region of higher frequencies of IR radiation. Graphs of this pattern are also available in various sources and I will not draw them. The second pattern was already truly amazing. It turned out that different substances at the same temperature have an emission peak at the same frequency. The situation required a theoretical explanation. And here Planck proposes a formula, linking the energy and frequency of radiation:
where E is energy, f is the frequency of radiation, and h is a constant value, which was later named after him. Planck also calculated the value of this quantity, which, according to his calculations, turned out to be equal to
h \u003d 6.626 * 10 -34 j * sec.
Quantitatively, this formula does not describe real experimental data quite accurately, and later you will see why, but from the point of view of a theoretical explanation of the situation, it is fully consistent with reality, which you will also see later.
Preparatory part
Next, we recall several physical laws that will form the basis of our further reasoning. The first will be the formula for the kinetic energy of a body that rotates along a circular or elliptical path. It looks like this:
those. the product of the mass of the body and the square of the speed with which the body moves in orbit. In this case, the speed V is calculated by a simple formula:
where T is the period of revolution, and R is taken as the radius of rotation for circular motion, and for an elliptical trajectory, the major semiaxis of the ellipse of the trajectory. For one atom of matter, there is one formula that is very useful for us, relating the temperature to the energy of the atom:
Here t is the temperature in degrees Kelvin, and k is the Boltzmann constant, which is 1.3807*10 -23 J/K. If we take the temperature to one degree, then, in accordance with this formula, the energy of one atom will be equal to:
(2) E = 4140*10 -26 J
Moreover, this energy will be the same for both an atom of lead and an atom of aluminum or an atom of any other chemical element. This is precisely the meaning of the concept of "temperature". From formula (1), which is valid for the solid and liquid state of aggregation of a substance, it can be seen that the equality of energies for different atoms with different masses at a temperature of 1 degree is achieved only by changing the value of the square of the velocity, i.e. the speed at which an atom moves in its circular or elliptical orbit. Therefore, knowing the energy of an atom at one degree and the mass of an atom, expressed in kilograms, we can easily calculate the linear velocity of a given atom at any temperature. Let us explain how this is done with a specific example. Let's take any chemical element from the periodic table, for example, molybdenum. Next, take any temperature, for example - 1000 degrees Kelvin. Knowing from formula (2) the value of the energy of an atom at 1 degree, we can find out the energy of an atom at the temperature we have taken, i.e. multiply this value by 1000. It turned out:
(3) Molybdenum atom energy at 1000K = 4.14*10 -20 J
Now we calculate the value of the mass of the molybdenum atom, expressed in kilograms. This is done using the periodic table. In the cell of each chemical element, near its serial number, its molar mass is indicated. For molybdenum, this is 95.94. It remains to divide this number by the Avogadro number, equal to 6.022 * 10 23 and multiply the result by 10 -3, since the molar mass is indicated in grams in the periodic table. It turns out 15.93 * 10 -26 kg. Further from the formula
mV 2 \u003d 4.14 * 10 -20 J
calculate the speed and get
V = 510m/sec.
Now it's time for us to move on to the next question of the preparatory material. Recall such a concept as angular momentum. This concept was introduced for bodies moving in a circle. A simple example can be taken: take a short tube, pass a cord through it, tie a load of mass m to the cord and, holding the cord with one hand, unwind the load above your head with the other hand. Multiplying the value of the speed of movement of the load by its mass and radius of rotation, we obtain the value of the angular momentum, which is usually denoted by the letter L. I.e.
By pulling the cord down through the tube, we will reduce the radius of rotation. In this case, the speed of rotation of the load will increase and its kinetic energy will increase by the amount of the work that you do by pulling the cord to reduce the radius. However, multiplying the mass of the load by the new values of speed and radius, we get the same value that we had before we reduced the radius of rotation. This is the law of conservation of momentum. Back in the 17th century, Kepler proved in his second law that this law is also observed for satellites moving around the planets in elliptical orbits. When approaching the planet, the speed of the satellite increases, and when moving away from it, it decreases. In this case, the mVR product remains unchanged. The same applies to the planets moving around the sun. In passing, we recall the third law of Kepler. You ask - why? Then, in this article you will see something that is not written in any scientific source - the formula of Kepler's third law of planetary motion in the microcosm. And now about the essence of this very third law. In the official interpretation, it sounds rather ornate: "the squares of the periods of revolutions of the planets around the Sun are proportional to the cubes of the semi-major axes of their elliptical orbits." Each planet has two personal parameters - the distance to the Sun and the time during which it makes one complete revolution around the Sun, i.e. circulation period. So, if you cube the distance, and then divide the result by the period squared, then you get some value, denote it by the letter C. And if you perform the above mathematical operations with the parameters of any other planet, you get the same value - C. Somewhat later, on the basis of Kepler's third law, Newton derived the Law of Universal Gravitation, and after another 100 years Cavendish calculated the true value of the gravitational constant - G. And only after that the true meaning of this very constant - C. It turned out that this is the encrypted value of the mass of the Sun, expressed in units of length cubed divided by time squared. Simply put, knowing the distance of the planet to the Sun and the period of its revolution, you can calculate the mass of the Sun. Skipping simple mathematical transformations, I will inform you that the conversion factor is equal to
Therefore, the formula is valid, with an analogue of which we will meet later:
(4) 4π 2 R 3 /T 2 G = M sun (kg)
Main part
Now we can move on to the main thing. Let's deal with the dimension of Planck's constant. From reference books we see that the value of Planck's constant
h \u003d 6.626 * 10 -34 j * sec.
For those who have forgotten physics, let me remind you that this dimension is equivalent to the dimension
kg * meter 2 / sec.
This is the dimension of the angular momentum
Now take the formula for the energy of an atom
and Planck's formula
For one atom of any substance at a given temperature, the values of these energies must be the same. Taking into account that the frequency is reciprocal to the period of radiation, i.e.
and the speed
where R is the radius of rotation of the atom, we can write:
m4π 2 R 2 /T 2 = h/T.
From this we see that Planck's constant is not pure angular momentum, but differs from it by a factor of 2π. Here we have determined its true essence. It remains only to calculate it. Before we start calculating it ourselves, let's see how others do it. Looking at the laboratory work on this topic, we will see that in most cases the Planck constant is calculated from the formulas for the photoelectric effect. But the laws of the photoelectric effect were discovered much later than Planck derived his constant. So let's look for another law. He is. This is Wien's law, discovered in 1893. The essence of this law is simple. As we have already said, at a certain temperature, a heated body has a peak in the intensity of IR radiation at a certain frequency. So, if you multiply the temperature value by the value of the IR radiation wave corresponding to this peak, you will get a certain value. If we take a different body temperature, then the radiation peak will correspond to a different wavelength. But even here, when multiplying these values, the same result will be obtained. Win calculated this constant and expressed his law as a formula:
(5) λt = 2.898*10 -3 m*degree K
Here λ is the IR wavelength in meters and t is the temperature in degrees Kelvin. This law in its significance can be equated with the laws of Kepler. Now, by looking at a heated body through a spectroscope and determining the wavelength at which the radiation peak is observed, it is possible to remotely determine the temperature of the body using the formula of Wien's law. All pyrometers and thermal imagers work on this principle. Although everything is not so simple here. The emission peak shows that most of the atoms in a heated body emit exactly this wavelength, i.e. have this temperature. And the radiation to the right and left of the peak shows that there are both “undercooled” and “overheated” atoms in the body. In real conditions, there are even several “humps” of radiation. Therefore, modern pyrometers measure the intensity of radiation at several points in the spectrum, and then the results are integrated, which makes it possible to obtain the most accurate results. But back to our questions. Knowing, on the one hand, that from formula (1) the temperature corresponds to the kinetic energy of the atom through a constant coefficient 3k, and on the other hand, the product of temperature and wavelength in Wien's law is also a constant, decomposing the square of the velocity in the formula for the kinetic energy of an atom into factors, we we can write:
m4π 2 R 2 λ/T 2 = constant.
On the left side of the equation, m is a constant, so everything else on the left side
4π 2 R 2 λ/T 2 is a constant.
Now compare this expression with the formula of Kepler's third law (4). Here, of course, we are not talking about the gravitational charge of the Sun, however, in this expression the value of a certain charge is encrypted, the essence and properties of which are very interesting. But this topic is worthy of a separate article, so we will continue our own. Let's calculate the value of Planck's constant using the example of the molybdenum atom, which we have already taken as an example. As we have already established, the formula for Planck's constant
Previously, we have already calculated the values of the mass of the molybdenum atom and the speed of its movement along its trajectory. We only need to calculate the radius of rotation. How to do it? This is where Wien's law comes in handy. Knowing the temperature value of molybdenum = 1000 degrees, we can easily calculate the wavelength λ using formula (5), which will turn out
λ \u003d 2.898 * 10 -6 m.
Knowing that infrared waves propagate in space at the speed of light - c, we use a simple formula
Let us calculate the radiation frequency of the molybdenum atom at a temperature of 1000 degrees. And this period will turn out
T \u003d 0.00966 * 10 -12 sec.
But this is exactly the frequency that the molybdenum atom generates, moving along its orbit of rotation. Previously, we have already calculated the speed of this movement V = 510 m / s, and now we also know the rotation frequency T. It remains only from a simple formula
calculate the radius of rotation R. It turns out
R \u003d 0.7845 * 10 -12 m.
And now we only have to calculate the value of Planck's constant, i.e. Multiply values
mass of an atom (15.93 * 10 -26 kg),
speed (510m/s),
radius of rotation (0.7845 * 10 -12 m)
and twice the value of pi. We get
4*10 -34 j*sec.
Stop! In any reference book you will find the value
6.626*10 -34 j*sec!
Who is right? Using this method, you yourself can calculate the value of Planck's constant for atoms of any chemical elements at any temperature not exceeding the evaporation temperature. In all cases, the value will be exactly
4*10 -34 j*sec,
6.626 * 10 -34 j * sec.
But. it is best that Planck himself give the answer to this question. Let's get into his formula
let's substitute our value for its constant, and we calculated the radiation frequency at 1000 degrees on the basis of Wien's law, which has been rechecked hundreds of times and has withstood all experimental tests. Given that the frequency is the reciprocal of the period, i.e.
Let's calculate the energy of a molybdenum atom at 1000 degrees. We get
4 * 10 -34 / 0.00966 * 10 -12 \u003d 4.14 * 10 -20 j.
And now let's compare the result obtained with another one obtained by an independent formula, the reliability of which is beyond doubt (3). These results match, which is the best evidence. And we will answer the last question - does Planck's formula contain irrefutable evidence that energy is transmitted only by quanta? Sometimes you read such an explanation in serious sources - you see, at a frequency of 1 Hz we have a certain value of energy, and at a frequency of 2 Hz it will be a multiple of Planck's constant. This is the quantum. Lord! The frequency value can be 0.15 Hz, 2.25 Hz or whatever. Frequency is an inverse function of wavelength and, for electromagnetic radiation, is related via the speed of light by a function like
The graph of this function does not allow any quantization. And now about quanta in general. In physics, there are laws expressed in formulas, where there are whole indivisible numbers. For example, the electrochemical equivalent is calculated by the formula mass of an atom / k, where k is an integer equal to the valence of a chemical element. Integers are also present when capacitors are connected in parallel when calculating the total capacity of the system. It's the same with energy. The simplest example is the transition of a substance to a gaseous state, where a quantum in the form of the number 2 is uniquely present. The Balmer series and some other relationships are also interesting. But this has nothing to do with Planck's formula. By the way, Planck himself was of the same opinion.
Conclusion
If the discovery of Wien's law can be compared in importance with Kepler's laws, then Planck's discovery can be compared with the discovery of the Law of Universal Gravitation. He turned Wien's faceless constant into a constant that has both dimension and physical meaning. Having proved that in a liquid or solid aggregate state of matter, for atoms of any elements at any temperature, the angular momentum is preserved, Planck made a great discovery that allowed us to take a fresh look at the physical world around us. In conclusion, I will give an interesting formula derived from the above and combining four physical constants - the speed of light - c, Wien's constant - v, Planck's constant - h and Boltzmann's constant - k.
Memorial sign to Max Planck in honor of the discovery of the Planck constant, on the facade of the Humboldt University, Berlin. The inscription reads: “Max Planck, who invented the elementary quantum of action, taught in this building. h, from 1889 to 1928". - an elementary quantum of action, a fundamental physical quantity that reflects the quantum nature of the Universe. The total angular momentum of a physical system can only change by a multiple of Planck's constant. As far as in quantum mechanics, physical quantities are expressed in terms of Planck's constant.Planck's constant is denoted by the Latin letter h. It has the dimension of energy multiplied by time.
More commonly used Planck's summary constant
In addition to the fact that it is convenient for use in the formulas of quantum mechanics, it has a special designation, you can’t confuse it with anything.
In the SI system, Planck's constant has the following meaning:
For calculations in quantum physics, it is more convenient to use the value of Planck's summary constant, expressed in terms of electron volts.
Max Planck introduced his constant to explain the radiation spectrum of a completely black body, assuming that the body emits electromagnetic waves in portions (quanta) with an energy proportional to the frequency (h?). In 1905, Einstein used this assumption to explain the photoelectric effect by postulating that electromagnetic waves are absorbed in bursts of energy proportional to frequency. This is how quantum mechanics was born, the validity of which both Nobel Prize winners doubted all their lives.
PLANK CONSTANT
h, one of the universal numerical constants of nature, which is included in many formulas and physical laws that describe the behavior of matter and energy on a microscopic scale. The existence of this constant was established in 1900 by Professor of Physics at the University of Berlin M. Planck in a work that laid the foundations of quantum theory. They also gave a preliminary estimate of its magnitude. The currently accepted value of Planck's constant is (6.6260755 ± 0.00023)*10 -34 J*s. Planck made this discovery while trying to find a theoretical explanation for the spectrum of radiation emitted by heated bodies. Such radiation is emitted by all bodies consisting of a large number of atoms at any temperature above absolute zero, but it becomes noticeable only at temperatures close to the boiling point of water of 100 ° C and above it. In addition, it covers the entire spectrum of frequencies from the radio frequency range to the infrared, visible and ultraviolet regions. In the visible light region, the radiation becomes sufficiently bright only at approximately 550°C. The frequency dependence of the radiation intensity per unit time is characterized by the spectral distributions shown in Fig. 1 for multiple temperatures. The radiation intensity at a given frequency value is the amount of energy emitted in a narrow frequency band in the vicinity of a given frequency. The area of the curve is proportional to the total energy radiated at all frequencies. It is easy to see that this area increases rapidly with increasing temperature.
Planck wanted to theoretically derive the spectral distribution function and find an explanation for two simple experimental patterns: the frequency corresponding to the brightest glow of a heated body is proportional to the absolute temperature, and the total energy radiated for 1 with a unit area of the surface of a completely black body is the fourth power of its absolute temperature . The first regularity can be expressed by the formula
Where nm is the frequency corresponding to the maximum radiation intensity, T is the absolute temperature of the body, and a is a constant depending on the properties of the emitting object. The second regularity is expressed by the formula
Where E is the total energy emitted by a single surface area in 1 s, s is a constant characterizing the radiating object, and T is the absolute temperature of the body. The first formula is called the Wien displacement law, and the second is called the Stefan-Boltzmann law. Based on these laws, Planck sought to derive an exact expression for the spectral distribution of radiated energy at any temperature. The universal nature of the phenomenon could be explained from the standpoint of the second law of thermodynamics, according to which thermal processes occurring spontaneously in a physical system always go in the direction of establishing thermal equilibrium in the system. Imagine that two hollow bodies A and B of different shapes, different sizes and from different materials with the same temperature face each other, as shown in Fig. 2. If we assume that more radiation comes from A to B than from B to A, then the body B would inevitably become warmer due to A and the equilibrium would be spontaneously disturbed. This possibility is excluded by the second law of thermodynamics, and therefore, both bodies must radiate the same amount of energy, and, therefore, the value of s in formula (2) does not depend on the size and material of the radiating surface, provided that the latter is a kind of cavity. If the cavities were separated by a colored screen that would filter and reflect back all radiation except radiation with any one frequency, then everything said would remain true. This means that the amount of radiation emitted by each cavity in each section of the spectrum is the same, and the spectral distribution function for the cavity has the character of a universal law of nature, and the value a in formula (1), like the value s, is a universal physical constant.

Planck, who was well versed in thermodynamics, preferred just such a solution to the problem and, acting by trial and error, found a thermodynamic formula that allowed him to calculate the spectral distribution function. The resulting formula agreed with all available experimental data and, in particular, with empirical formulas (1) and (2). To explain this, Planck used a clever trick suggested by the second law of thermodynamics. Rightly believing that the thermodynamics of matter is better studied than the thermodynamics of radiation, he concentrated his attention mainly on the matter of the walls of the cavity, and not on the radiation inside it. Since the constants included in the laws of Wien and Stefan-Boltzmann do not depend on the nature of the substance, Planck was free to make any assumptions about the material of the walls. He chose a model in which the walls are composed of a huge number of tiny electrically charged oscillators, each with its own frequency. Oscillators under the action of radiation incident on them can oscillate, while radiating energy. The whole process could be investigated based on the well-known laws of electrodynamics, i.e. the spectral distribution function could be found by calculating the average energy of oscillators with different frequencies. Reversing the sequence of reasoning, Planck, based on the correct spectral distribution function he guessed, found a formula for the average energy U of an oscillator with a frequency n in a cavity that is in equilibrium at an absolute temperature T:
Where b is a quantity determined experimentally, and k is a constant (called the Boltzmann constant, although it was first introduced by Planck), which appears in thermodynamics and the kinetic theory of gases. Since this constant usually enters with a factor T, it is convenient to introduce a new constant h = bk. Then b = h/k and formula (3) can be rewritten as
The new constant h is Planck's constant; its value calculated by Planck was 6.55×10-34 JChs, which is only about 1% different from the modern value. Planck's theory made it possible to express the value of s in formula (2) in terms of h, k and the speed of light c:
![]()
This expression agreed with experiment to the extent that the constants were known; more accurate measurements later found no discrepancies. Thus, the problem of explaining the spectral distribution function has been reduced to a more "simple" problem. It was necessary to explain what is the physical meaning of the constant h, or rather the product hn. Planck's discovery was that its physical meaning can be explained only by introducing a completely new concept of "quantum of energy" into mechanics. On December 14, 1900, at a meeting of the German Physical Society, Planck in his report showed that formula (4), and thus the rest of the formulas, can be explained if we assume that an oscillator with a frequency n exchanges energy with an electromagnetic field not continuously, but, as it were, in steps, gaining and losing its energy in discrete portions, quanta, each of which is equal to hn.
see also
ELECTROMAGNETIC RADIATION ;
HEAT ;
THERMODYNAMICS.
The consequences of the discovery made by Planck are set out in the articles PHOTOELECTRIC EFFECT;
COMPTON EFFECT;
ATOM;
ATOM STRUCTURE;
QUANTUM MECHANICS . Quantum mechanics is a general theory of phenomena on the scale of the microcosm. Planck's discovery now appears as an important consequence of a special nature following from the equations of this theory. In particular, it turned out that it is valid for all energy exchange processes that occur during oscillatory motion, for example, in acoustics and in electromagnetic phenomena. This explains the high penetrating power of X-rays, whose frequencies are 100-10,000 times higher than the frequencies characteristic of visible light, and whose quanta have a correspondingly higher energy. Planck's discovery serves as the basis for the entire wave theory of matter dealing with the wave properties of elementary particles and their combinations. It is known from Maxwell's theory that a beam of light with energy E carries a momentum p equal to
Where c is the speed of light. If light quanta are considered as particles, each of which has an energy hn, then it is natural to assume that each of them has a momentum p equal to hn/c. The fundamental relation relating the wavelength l to the frequency n and the speed of light c has the form
So the expression for momentum can be written as h/l. In 1923, graduate student L. de Broglie suggested that not only light, but also all forms of matter, are characterized by wave-particle duality, expressed in the relationships
Between the characteristics of a wave and a particle. This hypothesis was confirmed, which made Planck's constant a universal physical constant. Her role turned out to be much more significant than one might have assumed from the very beginning.
LITERATURE
Quantum metrology and fundamental constants. M., 1973 Shepf H.-G. From Kirchhoff to Planck. M., 1981
Collier Encyclopedia. - Open Society. 2000 .
See what "PLANK CONSTANT" is in other dictionaries:
- (quantum of action) the main constant of quantum theory (see Quantum mechanics), named after M. Planck. Planck constant h ??6,626.10 34 J.s. The value is often used. \u003d h / 2???? 1.0546.10 34 J.s, which is also called Planck's constant ... Big Encyclopedic Dictionary
- (quantum of action, denoted by h), fundamental physical. a constant that defines a wide range of physical. phenomena for which the discreteness of quantities with the dimension of the action is essential (see QUANTUM MECHANICS). Introduced by him. physicist M. Planck in 1900 with ... ... Physical Encyclopedia
- (quantum of action), the main constant of quantum theory (see Quantum mechanics). Named after M. Planck. Planck constant h≈6.626 10 34 J s. The value h = h / 2π≈1.0546 10 34 J s is often used, also called the Planck constant. * * *… … encyclopedic Dictionary
Planck's constant (quantum of action) is the main constant of quantum theory, a coefficient that relates the magnitude of the energy of electromagnetic radiation to its frequency. It also has the meaning of an action quantum and an angular momentum quantum. Introduced into scientific use by M ... Wikipedia
Quantum of action (See. Action), a fundamental physical constant (See. Physical constants), which determines a wide range of physical phenomena for which the discreteness of action is essential. These phenomena are studied in quantum mechanics (See ... Great Soviet Encyclopedia
- (quantum of action), osn. constant of quantum theory (see Quantum mechanics). Named after M. Planck. P. p. h 6.626 * 10 34 J * s. The value H \u003d h / 2PI 1.0546 * 10 34 J * s is often used, also called. P. p ... Natural science. encyclopedic Dictionary
Fundamental physics. constant, quantum of action, having the dimension of the product of energy and time. Defines a physical phenomena of the microworld, for which discrete physical is characteristic. quantities with the dimension of action (see Quantum mechanics). In size... ... Chemical Encyclopedia
One of the absolute physical constants, which has the dimension of action (energy X time); in the CGS system, the P. p. h is (6.62377 + 0.00018). 10 27 erg x sec (+0.00018 possible measurement error). It was first introduced by M. Planck (M. Planck, 1900) in ... ... Mathematical Encyclopedia
Quantum of action, one of the main. constants of physics, reflects the specifics of regularities in the microcosm and plays a fundamental role in quantum mechanics. P. p. h (6.626 0755 ± 0.000 0040) * 10 34 J * s. Often use the value L \u003d d / 2n \u003d (1.054 572 66 ± ... Big encyclopedic polytechnic dictionary
Plank constant (quantum of action)- one of the fundamental world constants (constants), which plays a decisive role in the microworld, manifested in the existence of discrete properties of micro-objects and their systems, expressed in integer quantum numbers, with the exception of half-integers ... ... Beginnings of modern natural science
Books
- Universe and physics without "dark energy" (discoveries, ideas, hypotheses). In 2 volumes. Volume 1, O. G. Smirnov. The books are devoted to the problems of physics and astronomy that have existed in science for decades and hundreds of years from G. Galileo, I. Newton, A. Einstein to the present day. The smallest particles of matter and planets, stars and ...