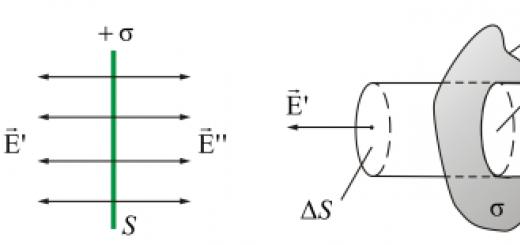
An electron is an elementary particle, which is one of the main units in the structure of matter. The charge of an electron is negative. The most accurate measurements were made in the early twentieth century by Millikan and Ioffe.
The electron charge is equal to minus 1.602176487 (40) * 10 -1 9 C.
Through this value, the electric charge of other smallest particles is measured.
General concept of the electron
In particle physics, it is said that the electron is indivisible and has no structure. It is involved in electromagnetic and gravitational processes, belongs to the lepton group, just like its antiparticle, the positron. Among other leptons, it has the lightest weight. If electrons and positrons collide, this leads to their annihilation. Such a pair can arise from the gamma-quantum of particles.
Before the neutrino was measured, it was the electron that was considered the lightest particle. In quantum mechanics, it is referred to as fermions. The electron also has a magnetic moment. If a positron is also referred to it, then the positron is separated as a positively charged particle, and the electron is called a negatron, as a particle with a negative charge.
 «>
«>
Individual properties of electrons
Electrons belong to the first generation of leptons, with the properties of particles and waves. Each of them is endowed with a quantum state, which is determined by measuring the energy, spin orientation, and other parameters. His belonging to fermions is revealed through the impossibility of two electrons being in the same quantum state at the same time (according to the Pauli principle).
It is studied in the same way as a quasiparticle in a periodic crystal potential, in which the effective mass can differ significantly from the mass at rest.
Through the movement of electrons, an electric current, magnetism and thermo EMF occur. The charge of an electron in motion forms a magnetic field. However, an external magnetic field deflects the particle from a straight direction. When accelerated, the electron acquires the ability to absorb or emit energy as a photon. Its set consists of electron atomic shells, the number and position of which determine the chemical properties.
The atomic mass mainly consists of nuclear protons and neutrons, while the mass of electrons is about 0.06% of the total atomic weight. The Coulomb electric force is one of the main forces that can keep an electron close to the nucleus. But when molecules are created from atoms and chemical bonds arise, electrons are redistributed in the new space formed.
Nucleons and hadrons are involved in the appearance of electrons. Isotopes with radioactive properties are capable of emitting electrons. Under laboratory conditions, these particles can be studied in special instruments, and, for example, telescopes can detect radiation from them in plasma clouds.
Opening
The electron was discovered by German physicists in the nineteenth century, when they studied the cathodic properties of rays. Then other scientists began to study it in more detail, bringing it to the rank of a separate particle. Radiation and other related physical phenomena were studied.
For example, a group led by Thomson estimated the charge of an electron and the mass of cathode rays, the ratios of which, as they found out, do not depend on a material source.
And Becquerel found that minerals emit radiation by themselves, and their beta rays can be deflected by the action of an electric field, while mass and charge retained the same ratio as that of cathode rays.
Atomic theory
According to this theory, an atom consists of a nucleus and electrons around it, arranged in the form of a cloud. They are in some quantized states of energy, the change of which is accompanied by the process of absorption or emission of photons.
Quantum mechanics
At the beginning of the twentieth century, a hypothesis was formulated, according to which material particles have the properties of both proper particles and waves. Also, light can manifest itself in the form of a wave (it is called the de Broglie wave) and particles (photons).
As a result, the famous Schrödinger equation was formulated, which described the propagation of electron waves. This approach is called quantum mechanics. It was used to calculate the electronic states of energy in the hydrogen atom.
Fundamental and quantum properties of the electron
The particle exhibits fundamental and quantum properties.
The fundamental ones include mass (9.109 * 10 -31 kilograms), elementary electric charge (that is, the minimum portion of the charge). According to the measurements that have been carried out so far, no elements are found in the electron that can reveal its substructure. But some scientists are of the opinion that it is a point charged particle. As indicated at the beginning of the article, the electronic electric charge is -1.602 * 10 -19 C.
“> Being a particle, an electron can simultaneously be a wave. The experiment with two slits confirms the possibility of its simultaneous passage through both of them. This conflicts with the properties of the particle, where it is only possible to pass through one slit each time.
Electrons are considered to have the same physical properties. Therefore, their permutation, from the point of view of quantum mechanics, does not lead to a change in the system state. The wave function of electrons is antisymmetric. Therefore, its solutions vanish when identical electrons enter the same quantum state (Pauli's principle).
Electron. Formation and structure of the electron. Magnetic monopole of an electron.
(continuation)
Part 4. The structure of the electron.
4.1. The electron is a two-component particle, which consists of only two super-condensed (condensed, concentrated) fields - the electric field-minus and the magnetic field-N. Wherein:
a) electron density - the maximum possible in Nature;
b) electron dimensions (D = 10 -17 cm and less) are minimal in Nature;
c) in accordance with the requirement of energy minimization, all particles - electrons, positrons, particles with a fractional charge, protons, neutrons, etc. must have (and have) a spherical shape;
d) for unknown reasons, regardless of the energy value of the "parent" photon, absolutely all electrons (and positrons) are born absolutely identical in their parameters (for example, the mass of absolutely all electrons and positrons is 0.511 MeV).
4.2. “It is reliably established that the magnetic field of an electron is the same integral property as its mass and charge. The magnetic fields of all electrons are the same, just as their masses and charges are the same. ”(c) This automatically allows us to draw an unambiguous conclusion about the equivalence of the mass and charge of the electron, that is: the mass of the electron is the equivalent of the charge, and vice versa, the charge of the electron is the equivalent of the mass (for positron - similarly).
4.3. This equivalence property also applies to particles with fractional charges (+2/3) and (-1/3), which are the basis of quarks. That is: the mass of the positron, electron and all fractional particles is the equivalent of their charge, and vice versa - the charges of these particles are the equivalent of the mass. Therefore, the specific charge of the electron, positron and all fractional particles is the same (const) and is equal to 1.76 * 10 11 C/kg.
4.4. Since an elementary quantum of energy is automatically an elementary quantum of mass, the mass of an electron (taking into account the presence of fractional particles 1/3 and 2/3) must have values that are multiples of the masses of three negative semiquanta. (See also "Photon. The structure of the photon. The principle of movement. paragraph 3.4.)
4.5. It is very difficult to determine the internal structure of an electron for many reasons, however, it is of considerable interest, at least in the first approximation, to consider the influence of two components (electric and magnetic) on the internal structure of an electron. See fig. 7.

Fig.7. The internal structure of the electron, options:
Option number 1. Each pair of leaves of the negative half-quantum forms "microelectrons", which then form an electron. In this case, the number of "microelectrons" must be a multiple of three.
Option number 2. The electron is a two-component particle, which consists of two docked independent hemispherical monopoles - electric (-) and magnetic (N).
Option number 3. The electron is a two-component particle, which consists of two monopoles - electric and magnetic. In this case, the spherical magnetic monopole is located at the center of the electron.
Option number 4. Other options.
Apparently, a variant can be considered when electric (-) and magnetic fields (N) can exist inside an electron not only in the form of compact monopoles, but also in the form of a homogeneous substance, that is, they form a practically structureless? crystalline? homogeneous? particle. However, this is highly doubtful.
4.6. Each of the proposed options has its own advantages and disadvantages, for example:
a) Options #1. Electrons of this design make it possible to easily form fractional particles with a mass and charge that is a multiple of 1/3, but at the same time make it difficult to explain the electron's own magnetic field.
b) Option number 2. This electron, when moving around the nucleus of an atom, is constantly oriented to the nucleus with its electric monopole and therefore can have only two options for rotation around its axis - clockwise or counterclockwise (Pauli's prohibition?), etc.
4.7. When considering these (or newly proposed) options, it is imperative to take into account the actual properties and characteristics of the electron, as well as take into account a number of mandatory requirements, for example:
- the presence of an electric field (charge);
— the presence of a magnetic field;
- equivalence of some parameters, for example: the mass of an electron is equivalent to its charge and vice versa;
- the ability to form fractional particles with a mass and charge that is a multiple of 1/3;
- the presence of a set of quantum numbers, spin, etc.
4.8. The electron appeared as a two-component particle, in which one half (1/2) is a compacted electric field-minus (electric monopole-minus), and the second half (1/2) is a compacted magnetic field (magnetic monopole-N). However, it should be borne in mind that:
- electric and magnetic fields under certain conditions can generate each other (turn into each other);
- An electron cannot be a one-component particle and consist of 100% of the minus field, since a singly charged minus field will decay due to repulsive forces. That is why the presence of a magnetic component is necessary inside the electron.
4.9. Unfortunately, it is not possible to conduct a complete analysis of all the advantages and disadvantages of the proposed options and choose the only correct version of the internal structure of the electron in this work.
Part 5. "Wave properties of an electron".
5.1. By the end of 1924 the point of view according to which electromagnetic radiation behaves partly like waves, and partly like particles, became generally accepted ... And it was at this time that the Frenchman Louis de Broglie, who at that time was a graduate student, had a brilliant idea: why the same cannot be for substances? Louis de Broglie did the reverse work on particles that Einstein did on light waves. Einstein connected electromagnetic waves with particles of light; de Broglie associated the motion of particles with the propagation of waves, which he called waves of matter. De Broglie's hypothesis was based on the similarity of the equations describing the behavior of light rays and particles of matter, and was of an exclusively theoretical nature. To confirm or refute it, experimental facts were required. ”(c)
5.2. “In 1927, American physicists K.Davisson and K.Jermer discovered that when electrons are “reflection” from the surface of a nickel crystal, maxima appear at certain angles of reflection. Similar data (the appearance of maxima) were already available from the observation of the diffraction of x-ray waves by crystalline structures. Therefore, the appearance of these maxima in reflected electron beams could not be explained in any other way than on the basis of ideas about waves and their diffraction. Thus, the wave properties of particles - electrons (and de Broglie's hypothesis) were proved by experiment. ”(c)
5.3. However, consideration of the process of the appearance of corpuscular properties of a photon described in this paper (see Fig. 5.) allows us to draw quite unambiguous conclusions:
a) as the wavelength decreases from 10 -4 to 10 - 10 (C)(C)(C)(C)(C)cm The photon's electric and magnetic fields condense
(C) (C) (C) (C) (C) (C) (C) (C) (C) (C) and already in the x-ray range the field density is commensurate with the density of an "ordinary" particle.
c) therefore, an X-ray photon, when interacting with an obstacle, is no longer reflected from the obstacle as a wave, but begins to bounce off it as a particle.
5.4. I.e:
a) already in the range of soft X-rays, the electromagnetic fields of photons are so condensed that it is very difficult to detect their wave properties. Quote: "The smaller the wavelength of a photon, the more difficult it is to detect the properties of a wave in it and the more strongly the properties of a particle appear in it."
b) in the hard X-ray and gamma range, photons behave like 100% particles, and it is almost impossible to detect wave properties in them. That is: the X-ray and gamma-ray photon completely loses the properties of the wave and turns into a 100% particle. Quote: "The energy of quanta in the X-ray and gamma range is so great that the radiation behaves almost like a stream of particles" (c).
c) therefore, in experiments on the scattering of an X-ray photon from the surface of a crystal, it was no longer a wave that was observed, but an ordinary particle that bounced off the surface of the crystal and repeated the structure of the crystal lattice.
5.5. Prior to the experiments of K. Davisson and K. Germer, there were already experimental data on the observation of the diffraction of X-ray waves on crystal structures. Therefore, having obtained similar results in experiments with the scattering of electrons on a nickel crystal, they automatically attributed wave properties to the electron. However, an electron is a “solid” particle that has a real rest mass, dimensions, etc. It is not an electron-particle that behaves like a photon-wave, but an X-ray photon has (and exhibits) all the properties of a particle. Not an electron is reflected from an obstacle as a photon, but an X-ray photon is reflected from an obstacle as a particle.
5.6. Therefore: the electron (and other particles) did not have any “wave properties”, there is not and cannot be. And there are no prerequisites, much less opportunities to change this situation.
Part 6. Conclusions.
6.1. Electron and positron are the first and fundamental particles, the presence of which determined the appearance of quarks, protons, hydrogen and all other elements of the periodic table.
6.2. Historically, one particle was named an electron and given a minus sign (matter), and the other was called a positron and given a plus sign (antimatter). “The electric charge of the electron was agreed to be considered negative in accordance with an earlier agreement to call the charge of electrified amber negative” (c).
6.3. An electron can appear (appear = be born) only in a pair with a positron (an electron is a positron pair). The appearance in Nature of at least one "unpaired" (single) electron or positron is a violation of the law of conservation of charge, the general electroneutrality of matter and is technically impossible.
6.4. The formation of an electron-positron pair in the Coulomb field of a charged particle occurs after the separation of the elementary quanta of a photon in the longitudinal direction into two component parts: a negative one, from which a minus particle (electron) is formed, and a positive one, from which a plus particle (positron) is formed. The separation of an electrically neutral photon in the longitudinal direction into two absolutely equal in mass, but different in charges (and magnetic fields) parts is a natural property of the photon, which follows from the laws of charge conservation, etc. The presence of even negligible amounts of “particles-plus” “inside” the electron , and "inside" the positron - "minus particles" - is excluded. It also excludes the presence of electrically neutral "particles" (cuts, pieces, fragments, etc.) of the parent photon inside the electron and proton.
6.5. For unknown reasons, absolutely all electrons and positrons are born as reference "maximum-minimum" particles (i.e. they cannot be larger and cannot be smaller in mass, charge, dimensions and other characteristics). The formation of any smaller or larger particles-plus (positrons) and particles-minus (electrons) from electromagnetic photons is excluded.
6.6. The internal structure of the electron is unambiguously predetermined by the sequence of its appearance: the electron is formed as a two-component particle, which is 50% compacted electric field-minus (electric monopole-minus), and 50% compacted magnetic field (magnetic monopole-N). These two monopoles can be considered as differently charged particles, between which forces of mutual attraction (adhesion) arise.
6.7. Magnetic monopoles exist, but not in a free form, but only as components of an electron and a positron. In this case, the magnetic monopole-(N) is an integral part of the electron, and the magnetic monopole-(S) is an integral part of the positron. The presence of a magnetic component “inside” the electron is necessary, since only a magnetic monopole-(N) can form the strongest (and unprecedented in strength) bond with a singly charged electric monopole-minus.
6.8. Electrons and positrons have the greatest stability and are particles whose decay is theoretically and practically impossible. They are indivisible (by charge and mass), that is: spontaneous (or forced) separation of an electron or positron into several calibrated or “different-sized” parts is excluded.
6.9. The electron is eternal and it cannot “disappear” until it meets another particle with equal in magnitude but opposite in sign electric and magnetic charges (positron).
6.10. Since only two standard (calibrated) particles can appear from electromagnetic waves: an electron and a positron, then only standard quarks, protons and neutrons can appear on their basis. Therefore, all visible (baryonic) matter of our and all other universes consists of the same chemical elements (Mendeleev's table) and uniform physical constants and fundamental laws similar to "our" laws act everywhere. The appearance at any point of the infinite space of "other" elementary particles and "other" chemical elements is excluded.
6.11. All visible matter of our Universe was formed from photons (presumably in the microwave range) according to the only possible scheme: photon → electron-positron pair → fractional particles → quarks, gluon → proton (hydrogen). Therefore, all the "solid" matter of our Universe (including Homo sapiens) is condensed electric and magnetic fields of photons. There were no other “materials” for its formation in the Cosmos, and there cannot be.
P.S. Is the electron inexhaustible?
Properties
The electron charge is indivisible and is equal to −1.602176565(35) 10 −19 C (or −4.80320427(13) 10 −10 units of the CGSE charge in the CGSE system or −1.602176565(35) 10 −20 units .cgsm in the cgsm system); it was first directly measured in experiments ( English) A. F. Ioffe (1911) and R. Millikan (1912). This value serves as a unit of measurement of the electric charge of other elementary particles (unlike the charge of an electron, the elementary charge is usually taken with a positive sign). The electron mass is 9.10938291(40) 10 −31 kg.
Kg is the mass of the electron.
Cl is the charge of an electron.
C/kg - specific electron charge.
Electron spin in units
According to modern concepts of elementary particle physics, the electron is indivisible and structureless (at least up to distances of 10 −17 cm). The electron participates in weak, electromagnetic and gravitational interactions. It belongs to the group of leptons and is (together with its antiparticle, the positron) the lightest of the charged leptons. Before the discovery of the neutrino mass, the electron was considered the lightest of the massive particles - its mass is about 1836 times less than the mass of the proton. The spin of an electron is 1/2, and thus the electron is a fermion. Like any charged particle with spin, an electron has a magnetic moment, and the magnetic moment is divided into a normal part and an anomalous magnetic moment. Sometimes both electrons themselves and positrons are referred to as electrons (for example, considering them as a common electron-positron field, a solution of the Dirac equation). In this case, a negatively charged electron is called a negatron, a positively charged one is called a positron. [ source not specified 120 days]
Being in the periodic potential of the crystal, the electron is considered as a quasi-particle, the effective mass of which can differ significantly from the mass of the electron.
A free electron cannot absorb a photon, although it can scatter it (see the Compton effect).
Etymology and history of discovery
The name "electron" comes from the Greek word ἤλεκτρον, meaning "amber": back in ancient Greece, natural scientists conducted experiments - pieces of amber were rubbed with wool, after which they began to attract small objects to themselves. The term "electron" as the name of the fundamental indivisible unit of charge in electrochemistry was proposed by J. J. Stoney ( English) in 1894 (the unit itself was introduced by him in 1874). The discovery of the electron as a particle belongs to E. Wiechert and J. J. Thomson, who in 1897 established that the charge-to-mass ratio for cathode rays does not depend on the material of the source. (see Discovery of the electron)
Discovery of wave properties. According to the hypothesis of de Broglie (1924), the electron (like all other material micro-objects) has not only corpuscular, but also wave properties. The de Broglie wavelength of a nonrelativistic electron is , where is the speed of the electron. Accordingly, electrons, like light, can experience interference and diffraction. The wave properties of electrons were experimentally discovered in 1927 by the American physicists C. Davisson and L. Germer (Davisson-Germer Experiment) and independently by the English physicist JP Thomson.
Usage
Most sources of low-energy electrons use the phenomena of thermionic emission and photoelectron emission. High-energy, with energies from several keV to several MeV, electrons are emitted in the processes of beta decay and internal conversion of radioactive nuclei. The electrons emitted in beta decay are sometimes called beta particles or beta rays. Accelerators serve as sources of electrons with higher energy.
The movement of electrons in metals and semiconductors makes it easy to transfer and control energy; this is one of the foundations of modern civilization and is used almost everywhere in industry, communications, computer science, electronics, and in everyday life. The drift velocity of electrons in conductors is very low (~0.1-1 mm/s), but the electric field propagates at the speed of light. In this regard, the current in the entire circuit is established almost instantly.
Electron beams accelerated to high energies, for example, in linear accelerators, are one of the main means of studying the structure of atomic nuclei and the nature of elementary particles. A more prosaic application of electron beams are televisions and monitors with cathode ray tubes (kinescopes). The electron microscope also exploits the ability of electron beams to obey the laws of electron optics. Before the invention of transistors, almost all radio engineering and electronics were based on vacuum electron tubes, where the control of the movement of electrons in vacuum by electric (sometimes magnetic) fields is used. Electrovacuum devices (EVD) continue to be used to a limited extent in our time; the most common applications are magnetrons in microwave oven generators and the aforementioned cathode ray tubes (CRTs) in televisions and monitors.
The electron as a quasiparticle
If the electron is in a periodic potential, its motion is considered as the motion of a quasiparticle. Its states are described by a quasi-wave vector. The main dynamic characteristic in the case of a quadratic dispersion law is the effective mass, which can differ significantly from the mass of a free electron and, in the general case, is a tensor.
Electron and Universe
It is known that out of every 100 nucleons in the universe, 87 are protons and 13 are neutrons (the latter are mainly part of helium nuclei). To ensure the overall neutrality of matter, the number of protons and electrons must be the same. The density of the baryonic (observed by optical methods) mass, which consists mainly of nucleons, is fairly well known (one nucleon per 0.4 cubic meters). Taking into account the radius of the observable Universe (13.7 billion light years), we can calculate that the number of electrons in this volume is ~10 80 , which is comparable to large Dirac numbers.
see also
- Theory of a one-electron universe
- Electricity
- Electronics
- Photomultiplier
- Electric lamp
An electron is a negatively charged elementary particle belonging to the class of leptons (see Elementary particles), the carrier of the smallest known now mass and the smallest electric charge in nature. Opened in 1897 by the English scientist J. J. Thomson.
An electron is an integral part of an atom, the number of electrons in a neutral atom is equal to the atomic number, i.e., the number of protons in the nucleus.
The first accurate measurements of the electric charge of the electron were carried out in 1909-1913. American fiaik R. Milliken. The modern value of the absolute value of the elementary charge is e = (4.803242 ± 0.000014) 10 -10 CGSE units or approximately 1.6 10 -19 C. It is believed that this charge is indeed "elementary", i.e., it cannot be divided into parts, and the charges of any objects are its integer multiples. You may have heard of quarks with electric charges e/3 and 2e/3, but apparently they are tightly locked inside hadrons and do not exist in a free state. Together with the Planck constant ħ and the speed of light c, the elementary charge forms a dimensionless constant α = e 2 /ħc ≈ 1/137. The fine structure constant α is one of the most important parameters of quantum electrodynamics, it determines the intensity of electromagnetic interactions (the most accurate modern value is α -1 = 137.035963 ± 0.000015).
Electron mass m e \u003d (9.109534 ± 0.000047) 10 -28 g (in energy units ≈0.5 MeV / s 2). If the laws of conservation of energy and electric charge are valid, then any decays of the electron are forbidden, such as e - → ν e + γ, etc. Therefore, the electron is stable; experimentally obtained that the time of his life is not less than 10 22 years.
In 1925, American physicists S. Goudsmit and J. Uhlenbeck introduced the internal angular momentum of an electron - spin (s) to explain the features of atomic spectra. The spin of an electron is half of Planck's constant (ħ = 1.055 10 -34 J/s), but physicists usually say simply that the spin of an electron is 1/2:s = 1/2. The spin of an electron is associated with its own magnetic moment М = ge s(eħ/2m e c). The value eħ / 2m e c \u003d 9.274 10 -21 erg / G is called the Bohr magneton MB (this is the unit of measurement of the magnetic moment adopted in atomic and nuclear physics; here ħ is Planck's constant, e and m are the absolute value of the charge and mass of the electron, c is the speed Sveta); the numerical coefficient ge is the g factor of the electron. From Dirac's quantum-mechanical relativistic equation (1928), the value ge = 2 followed, i.e., the magnetic moment of an electron should have been equal to exactly one Bohr magneton.
However, in 1947 it was found in experiments that the magnetic moment is approximately 0.1% larger than the Bohr magneton. The explanation of this fact was given taking into account the vacuum polarization in quantum electrodynamics. Very laborious calculations gave the theoretical value g e = 2 (1.001159652460 ± 0.000000000148), which can be compared with modern (1981) experimental data:
for electron g e = 2 (1.001159652200 ± 0.000000000040) and positron g e = 2 (1.001159652222 ± 0.000000000050). The values are calculated and measured with an accuracy of up to twelve decimal places, and the accuracy of experimental work is higher than the accuracy of theoretical calculations. These are the most precise measurements in particle physics.
The features of the motion of electrons in atoms, subject to the equations of quantum mechanics, determine the optical, electrical, magnetic, chemical and mechanical properties of substances.
Electrons participate in electromagnetic, weak and gravitational interactions (see Unity of the Forces of Nature). So, due to the electromagnetic process, an annihilation of an electron and a positron occurs with the formation of two γ-quanta: e + + e - → γ + γ. High-energy electrons and positrons can also participate in other processes of electromagnetic annihilation with the formation of hadrons: e + + e - hadrons. Now such reactions are intensively studied at numerous accelerators on colliding e + e - - beams (see Accelerators of Charged Particles).
Weak interactions of electrons manifest themselves, for example, in processes with parity nonconservation (see Parity) in atomic spectra or in reactions between electrons and neutrinos ν μ μ + e - → ν μ μ + e - .
There is no data on the internal structure of the electron. Modern theories proceed from the concept of leptons as point particles. At present, this has been experimentally verified up to distances of 10 -16 cm. New data may appear only with an increase in the particle collision energy in future accelerators.
An electron is an elementary particle that has a negative electrical charge. It is equal to -1. An electron is a part of all atoms, and therefore, of any substance. An electron is the lightest electrically charged particle. Electrons are usually denoted "e -".
What is important to know about electrons
In a metal, some of the electrons can move freely because they are not bound to atoms, which makes metals good conductors of electricity. Due to its small mass, the electron is the particle most involved in the development of the special theory of relativity, quantum mechanics, relativistic quantum field theory.
It is generally accepted that in our time the equations that describe the behavior of electrons in all physical conditions are fully known. All electrons obey Dirac-Fermi statistics. This is expressed in the Pauli principle, according to which two electrons cannot be in the same quantum state.
One consequence of this principle is that the states of the valence electrons (the most loosely bound electrons), which determine the chemical properties of atoms, depend on the charge number (atomic number), which is equal to the number of electrons in the atom.
Another consequence is that the "clouds" of electrons that envelop the nuclei of atoms have a resistance to their overlap. As a result, matter tends to occupy a certain space. Now you know what an electron is, but what are its characteristics?
Electron Characteristics
As it should be for all elementary particles, the number of basic characteristics of an electron is small:
- Mass (me, measured in MeV or grams);
- Charge (?e, measured in C);
- Spin (1/2ћ, measured in J·s, where ћ is Planck's constant h divided by 2).
Through these characteristics, all other characteristics of electrons are also expressed, for example, the magnetic moment, measured in J / T.
The structure of the electron
The structure of an electron is similar to the structure of an atom. An electron consists of a negatively charged shell and a positively charged nucleus (the mass of this particle).
The nucleus of an electron consists of electron antineutrinos (the positive charge of the nucleus). The electron shell consists of photons.
In the electron shell, the number of photons is greater than the number of antineutrinos in the nucleus. Since the electron has an excess of negative charge, it is negatively charged. A neutrino is also a composite particle, which is a bound state of a photon and a graviton.
Now you know everything about what an electron is!
Electron (elementary particle)
This article was written by Vladimir Gorunovich for the site "Wikiknowledge", under the title "Electron in the field theory", placed on this site in order to protect information from vandals, and then supplemented on this site.
The field theory of elementary particles, acting within the framework of SCIENCE, relies on a foundation proven by PHYSICS:
- classical electrodynamics,
- quantum mechanics,
- Conservation laws are the fundamental laws of physics.
This is the fundamental difference between the scientific approach used by the field theory of elementary particles - a true theory must strictly operate within the laws of nature: this is what SCIENCE is all about.
To use elementary particles that do not exist in nature, to invent fundamental interactions that do not exist in nature, or to replace the interactions that exist in nature with fabulous interactions, to ignore the laws of nature, doing mathematical manipulations on them (creating the appearance of science) - this is the lot of FAIRY TALES masquerading as science. As a result, physics slipped into the world of mathematical fairy tales.
1 Electron radius
2 Electron electric field
3 Electron magnetic moment
4 Rest mass of an electron
5 Physics of the 21st century: Electron (elementary particle) - result
Electron(Eng. Electron) - the lightest elementary particle with an electric charge. Quantum number L=1/2 (spin = 1/2) - leptons group, electron subgroup, electric charge -e (systematization according to the field theory of elementary particles). The stability of the electron is due to the presence of an electric charge, in the absence of which the electron would decay similarly to the muon neutrino.
According to the field theory of elementary particles, an electron consists of a rotating polarized alternating electromagnetic field with a constant component.
The structure of the electromagnetic field of an electron(E-constant electric field, H-constant magnetic field, yellow color indicates alternating electromagnetic field)
Energy balance (percentage of total internal energy):
- constant electric field (E) - 0.75%,
- permanent magnetic field (H) - 1.8%,
- alternating electromagnetic field - 97.45%.
This explains the pronounced wave properties of the electron and its unwillingness to participate in nuclear interactions. The structure of the electron is shown in the figure.
1 Electron radius
The radius of an electron (the distance from the center of the particle to the place where the maximum mass density is reached) is determined by the formula:

equal to 1.98 ∙10 -11 cm.
Occupied by an electron, determined by the formula:

is equal to 3.96 ∙10 -11 cm. The radius of the annular region occupied by the alternating electromagnetic field of the electron has been added to the value of r 0~. It must be remembered that part of the value of the rest mass, concentrated in the constant (electric and magnetic) fields of the electron is outside this region, in accordance with the laws of electrodynamics.
An electron is larger than any atomic nucleus, therefore it cannot be present in atomic nuclei, but is born in the process of neutron decay, just as a positron is born in the process of decay in a proton nucleus.
Statements that the radius of an electron is about 10 -16 cm are unsubstantiated and contradict classical electrodynamics. With such linear dimensions, the electron must be heavier than the proton.
2 Electron electric field
The electric field of an electron consists of two regions: an outer region with a negative charge and an inner region with a positive charge. The size of the inner region is determined by the radius of the electron. The difference between the charges of the outer and inner regions determines the total electric charge of the electron -e. Its quantization is based on the geometry and structure of elementary particles.
the electric field of an electron at point (A) in the far zone (r>> r e) exactly, in the SI system is:
electric field of an electron in the far zone (r > > r e) exactly, in the SI system is equal to:
where n= r/|r| - unit vector from the center of the electron in the direction of the observation point (A), r - distance from the center of the electron to the observation point, e - elementary electric charge, vectors are in bold type, ε 0 - electrical constant, r e \u003d Lħ / (m 0~ c ) is the radius of an electron in field theory, L is the main quantum number of an electron in field theory, ħ is Planck's constant, m 0~ is the mass of an electron at rest in an alternating electromagnetic field, c is the speed of light. (There is no multiplier in the CGS system.)
These mathematical expressions are correct for the far zone of the electric field of the electron: (r>>re), and the allegations that "the electric field of the electron remains Coulomb up to distances of 10 -16 cm" have nothing to do with reality - this is one of the fairy tales that contradicts the classical electrodynamics.
According to the field theory of elementary particles, a constant electric field of elementary particles with a quantum number L>0, both charged and neutral, is created by a constant component of the electromagnetic field of the corresponding elementary particle. And the field of electric charge arises as a result of the presence of asymmetry between the outer and inner hemispheres, generating electric fields of opposite signs. For charged elementary particles in the far zone, the field of an elementary electric charge is generated, and the sign of the electric charge is determined by the sign of the electric field generated by the outer hemisphere. In the near zone, this field has a complex structure and is a dipole, but it does not have a dipole moment. For an approximate description of this field as a system of point charges, at least 6 "quarks" inside the electron are required - it is better if we take 8 "quarks". It is clear that this is outside the scope of the standard model.
An electron, like any other charged elementary particle, has two electric charges and, accordingly, two electric radii:
- electric radius of external constant electric field (charge -1.25e) - r q- = 3.66 10 -11 cm.
- electric radius of internal constant electric field (charge +0.25e) - r q+ = 3 10 -12 cm.
These characteristics of the electron electric field correspond to distribution 1 of the field theory of elementary particles. Physics has not yet experimentally established the accuracy of this distribution, and which distribution most accurately corresponds to the real structure of the constant electric field of an electron in the near zone.
The electric radius indicates the average location of an electric charge evenly distributed around the circumference, which creates a similar electric field. Both electric charges lie in the same plane (the plane of rotation of the variable electromagnetic field of the elementary particle) and have a common center coinciding with the center of rotation of the variable electromagnetic field of the elementary particle.
Intensity E of the electric field of an electron in the near zone(r ~ r e), in the SI system, as a vector sum, is approximately equal to:
where n-=r-/r - unit vector from the near (1) or far (2) charge point q - electron in the direction of the observation point (A), n+=r+/r - unit vector from the near (1) or far (2) charge point q + electron in the direction of the observation point (A), r - distance from the center of the electron to the projection of the observation point onto the electron plane, q - - external electric charge -1.25 e, q + - internal electric charge +0.25e, vectors are in bold type, ε 0 - electrical constant, z - observation point height (A) (distance from the observation point to the electron plane), r 0 - normalization parameter. (There is no multiplier in the CGS system.)
This mathematical expression is the sum of vectors and it must be calculated according to the rules of vector addition, since this is a field of two distributed electric charges (q - = -1.25e and q + = +0.25e). The first and third terms correspond to the near points of the charges, the second and fourth - to the far ones. This mathematical expression does not work in the internal (ring) region of the electron, which generates its constant fields (if two conditions are simultaneously met: r
Electron electric field potential at point (A) in the near zone(r ~ r e), in the SI system is approximately equal to:
where r 0 is a normalization parameter, the value of which may differ from in the formula E. (There is no multiplier in the CGS system.) This mathematical expression does not work in the inner (ring) region of the electron, which generates its constant fields (if both conditions are met simultaneously: r
Calibration of r 0 for both expressions of the near zone must be performed at the boundary of the region that generates constant electron fields.
3 Electron magnetic moment
In contrast to quantum theory, the field theory of elementary particles states that the magnetic fields of elementary particles are not created by the spin rotation of electric charges, but exist simultaneously with a constant electric field as a constant component of the electromagnetic field. Therefore, all elementary particles with quantum number L>0 have magnetic fields.
Since the values of the principal quantum number L and the spin of leptons coincide, the values of the magnetic moments of charged leptons in both theories can also coincide.
The field theory of elementary particles does not consider the magnetic moment of an electron to be anomalous - its value is determined by a set of quantum numbers to the extent that quantum mechanics works in an elementary particle.
So, the main magnetic moment of an electron is created by a current:
- (-) with magnetic moment -0.5 eħ/m 0e s
To obtain the resulting magnetic moment of an electron, it is necessary to multiply by the percentage of the energy of the alternating electromagnetic field divided by 100 percent and add the spin component (see Field Theory of Elementary Particles source), as a result we get 0.5005786 eħ/m 0e c. In order to convert to ordinary Bohr magnetons, the resulting number must be multiplied by two.
4 Rest mass of an electron
In accordance with classical electrodynamics and Einstein's formula, the rest mass of elementary particles with quantum number L>0, including the electron, is defined as the energy equivalent of their electromagnetic fields:
where the definite integral is taken over the entire electromagnetic field of the elementary particle, E is the electric field strength, H is the magnetic field strength. Here all components of the electromagnetic field are taken into account: a constant electric field, a constant magnetic field, an alternating electromagnetic field.
As follows from the above formula, the value of the rest mass of an electron depends on the conditions in which the electron is located. So by placing an electron in a constant external electric field, we will affect E 2 , which will be reflected in the mass of the particle. A similar situation will arise when an electron is placed in a constant magnetic field.
5 Physics of the 21st century: Electron (elementary particle) - summary
A new world has opened before you - the world of dipole fields, the existence of which the physics of the 20th century did not suspect. You have seen that an electron has not one, but two electric charges (external and internal) and their corresponding two electric radii. You have seen that the linear dimensions of the electron are much larger than the linear dimensions of the proton. You have seen what makes up the rest mass of the electron and that the imaginary Higgs boson was out of work (the decisions of the Nobel Committee are not yet the laws of nature...). Moreover, the magnitude of the mass depends on the fields in which the electron is located. All this goes beyond the concepts that dominated physics in the second half of the twentieth century. - Physics of the 21st century - New physics moves to a new level of knowledge of matter.
Vladimir Gorunovich
This term has other meanings, see Electron (meanings). Electron 2 Electron is a series of four Soviet artificial Earth satellites launched in 1964. Purpose ... Wikipedia
Electron- (Novosibirsk, Russia) Hotel category: 3 star hotel Address: 2nd Krasnodonsky lane … Hotel catalog
- (symbol e, e), the first element. h tsa, discovered in physics; mater. the carrier of the smallest mass and the smallest electric. charge in nature. E. an integral part of atoms; their number in neutral. atom is equal to at. number, i.e., the number of protons in the nucleus. Charge (e) and mass ... ... Physical Encyclopedia
Electron- (Moscow, Russia) Hotel category: 2 star hotel Address: Andropov Prospekt 38 building 2 … Hotel catalog
Electron- (e, e) (from the Greek elektron amber; a substance that is easily electrified by friction), a stable elementary particle with a negative electric charge e=1.6´10 19 C and a mass of 9´10 28 g. Belongs to the class of leptons. Discovered by an English physicist ... ... Illustrated Encyclopedic Dictionary
- (e e), stable negatively charged elementary particle with spin 1/2, mass approx. 9.10 28 g and a magnetic moment equal to the Bohr magneton; refers to leptons and participates in electromagnetic, weak and gravitational interactions. ... ...
- (designation e), a stable ELEMENTARY PARTILE with a negative charge and a rest mass of 9.1310 31 kg (which is 1/1836 of the mass of the PROTON). Electrons were discovered in 1879 by the English physicist Joseph Thomson. They move around the CORE, ... ... Scientific and technical encyclopedic dictionary
Exist., number of synonyms: 12 delta electron (1) lepton (7) mineral (5627) ... Synonym dictionary
An artificial Earth satellite created in the USSR to study the radiation belts and the Earth's magnetic field. They were launched in pairs, one along a trajectory lying below and the other above the radiation belts. In 1964, 2 pairs of Electrons were launched ... Big Encyclopedic Dictionary
ELECTRON, elktron, husband. (Greek elektron amber). 1. A particle with the smallest negative electric charge that forms an atom in conjunction with a proton (physical). The movement of electrons creates an electric current. 2. only units Lightweight magnesium alloy, ... ... Explanatory Dictionary of Ushakov
ELECTRON, a, m. (special). The elementary particle with the smallest negative electric charge. Explanatory dictionary of Ozhegov. S.I. Ozhegov, N.Yu. Shvedova. 1949 1992 ... Explanatory dictionary of Ozhegov
Books
- Electron. Energy of Cosmos, Landau Lev Davidovich, Kitaigorodsky Alexander Isaakovich. Books by Nobel Prize winner Lev Landau and Alexander Kitaygorodsky are texts that turn the narrow-minded view of the world around. Most of us are constantly confronted with...
- Electron Space Energy, Landau L., Kitaigorodsky A.. Books by Nobel Prize winner Lev Landau and Alexander Kitaigorodsky texts that turn the philistine idea of the world around. Most of us are constantly confronted with...