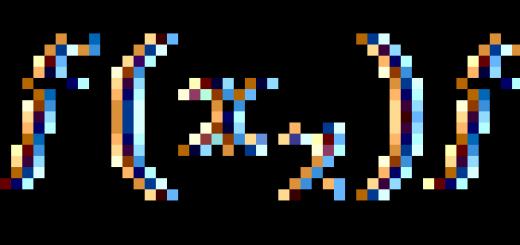Intrinsic mechanical and magnetic moments (spin)
JUSTIFICATION OF THE EXISTENCE OF SPIN. The Schrödinger equation makes it possible to calculate the energy spectrum of hydrogen and more complex atoms. However, the experimental determination of the energy levels of atoms showed that there is no complete agreement between theory and experiment. Precise measurements revealed the fine structure of the levels. All levels, except for the main one, are split into a number of very close sublevels. In particular, the first excited level of the hydrogen atom ( n= 2) split into two sublevels with an energy difference of only 4.5 10 -5 eV. For heavy atoms, the value of fine splitting is much greater than for light ones.
It was possible to explain this discrepancy between theory and experiment using the assumption (Uhlenbeck, Goudsmit, 1925) that the electron has one more internal degree of freedom - spin. According to this assumption, the electron and most of the others elementary particles along with the orbital angular momentum, they also have their own mechanical angular momentum. This proper moment is called spin.
The presence of spin in a microparticle means that in some respects it is like a small spinning top. However, this analogy is purely formal, since quantum laws significantly change the properties of the angular momentum. According to quantum theory, a point microparticle can have its own moment. An important and non-trivial quantum property of the spin is that only it can specify a preferred orientation in a particle.
The presence of an intrinsic mechanical moment in electrically charged particles leads to the appearance of their intrinsic (spin) magnetic moment, which, depending on the sign of the charge, is directed parallel (positive charge) or antiparallel (negative charge) to the spin vector. A neutral particle, for example, a neutron, can also have its own magnetic moment.
The existence of a spin in an electron was indicated by the experiments of Stern and Gerlach (1922) on observing the splitting of a narrow beam of silver atoms under the action of an inhomogeneous magnetic field(in a homogeneous field, the moment only changes orientation; only in an inhomogeneous field does it move forward either along the field or against it, depending on the direction with respect to the field). Unexcited silver atoms are in a spherically symmetrical s-state, that is, with an orbital momentum equal to zero. The magnetic moment of the system associated with the orbital motion of an electron (as in the classical theory) is directly proportional to the mechanical moment. If the latter is zero, then the magnetic moment must also be zero. This means that an external magnetic field should not affect the movement of silver atoms in the ground state. Experience shows that such an influence exists.
In the experiment, a beam of silver atoms was split, alkali metals and hydrogen, but always observed only two beams, equally deflected in opposite directions and located symmetrically with respect to the beam in the absence of a magnetic field. This can only be explained by the fact that the magnetic moment of a valence electron in the presence of a field can take on two values, identical in absolute value and opposite in sign.
The experimental results lead to the conclusion that the splitting in a magnetic field of a beam of atoms of the first group Periodic system, which are obviously in the s-state, into two components is explained by two possible states of the spin magnetic moment of the valence electron. The value of the projection of the magnetic moment on the direction of the magnetic field (it is this that determines the deflection effect), found from the experiments of Stern and Gerlach, turned out to be equal to the so-called Bohr magneton
The fine structure of the energy levels of atoms with one valence electron is explained by the presence of a spin in the electron as follows. in atoms (excluding s-states) due to orbital motion, there are electric currents, the magnetic field of which affects the spin magnetic moment (the so-called spin-orbit interaction). The magnetic moment of an electron can be oriented either along the field or against the field. States with different spin orientations differ somewhat in energy, which leads to the splitting of each level into two. Atoms with multiple electrons in the outer shell will have a more complex fine structure. So, for helium, which has two electrons, there are single lines (singlets) in the case of antiparallel electron spins (the total spin is zero - parahelium) and triple (triplets) in the case of parallel spins (the total spin is equal to h- orthohelium), which correspond to three possible projections on the direction of the magnetic field of the orbital currents of the total spin of two electrons (+h, 0, -h).
Thus, a number of facts led to the need to assign a new internal degree of freedom to electrons. For full description state, along with three coordinates or any other triplet of quantities that make up the quantum mechanical set, it is also necessary to set the value of the spin projection onto the chosen direction (the spin modulus does not need to be indicated, because, as experience shows, it does not change for any particle under any circumstances) .
The spin projection, as well as the projection of the orbital momentum, can change by a multiple of h. Since only two orientations of the electron spin were observed, Uhlenbeck and Goudsmit suggested that the projection of the electron spin S z in any direction can take two values: S z = ±h/2.
In 1928, Dirac obtained a relativistic quantum equation for the electron, from which the existence and spin of the electron follows h/2 without any special hypotheses.
The proton and neutron have the same spin 1/2 as the electron. The spin of a photon is equal to 1. But since the mass of a photon is equal to zero, then two, and not three of its projections +1 and -1 are possible. These two projections in Maxwell's electrodynamics correspond to two possible circular polarizations electromagnetic wave clockwise and counterclockwise relative to the direction of propagation.
PROPERTIES OF TOTAL PULSE TORQUE. Both the orbital moment M and the spin moment S are quantities that take only quantum discrete values. Consider now the total angular momentum, which is the vector sum of the mentioned moments.
The operator of the total angular momentum is defined as the sum of the operators and
The operators and commute, since the operator acts on the coordinates, while the operator does not act on them. It can be shown that
that is, the projections of the total angular momentum do not commute with each other in the same way as the projections of the orbital angular momentum. The operator, on the other hand, commutes with any projection, whence it follows that the operator and operator of any (but one) projection correspond to physical quantities and related to the number of simultaneously measurable. The operator also commutes with the and operators.
We determined the state of an electron in the field of the central force by three quantum numbers: n,l,m. quantum levels E n were generally determined by two quantum numbers n,l. In this case, the electron spin was not taken into account. If we also take into account the spin, then each state turns out to be essentially double, since two spin orientations are possible S z = hm s ; m s = ±1/2. Thus, a fourth is added to the three quantum numbers. m s, that is, the wave function, taking into account the spin, must be denoted.
For each term E n, l we have (2 l+ 1) states that differ in the orientation of the orbital momentum (the number m), each of which in turn splits into two states that differ in spin. Thus, there are 2(2 l+ 1) -fold degeneracy.
If we now take into account the weak interaction of the spin with the magnetic field of the orbital currents, then the energy of the state will also depend on the orientation of the spin with respect to the orbital momentum. The change in energy during such an interaction is small compared to the energy difference between levels with different n, l and therefore the emerging new lines are close to each other.
Thus, the difference in the orientations of the spin moment with respect to the internal magnetic field of the atom can explain the origin of the multiplicity of spectral lines. It follows from the foregoing that for atoms with one optical electron, only doublets (double lines) are possible due to two orientations of the electron spin. This conclusion is confirmed by experimental data. Let us now turn to the numbering of the levels of the atom, taking into account the multiplet structure. When the spin-orbit interaction is taken into account, neither the orbital momentum nor the spin momentum have a definite value in a state with a certain energy (the operators and do not commute with the operator). According to classical mechanics, we would have a precession of vectors and around the total moment vector, as shown in Fig. 20. The total moment remains constant. Similar provision also occurs in quantum mechanics. When the spin interaction is taken into account, only the total moment has a certain value in a state with a given energy (the operator commutes with the operator). Therefore, when taking into account the spin-orbit interaction, the state should be classified according to the value of the total momentum. The total momentum is quantized according to the same rules as the orbital momentum. Namely, if we introduce the quantum number j, which specifies the moment J, then
A projection on some direction 0 z has the meaning J z = hm j, wherein j= l + l s (l s= S) if the spin is parallel to the orbital moment, and j= | l- l s| if they are antiparallel. In a similar way m j = m+m s (m s= ±1/2). Since l,m are integers, and l s ,l m- halves, then
j = 1/2, 3/2, 5/2, … ; m j= ±1/2, ±3/2, … , ± j.

Depending on the orientation of the spin, the energy of the term will be different, namely, it will be for j = l+ S and j = |l- S|. Therefore, in this case, the energy levels should be characterized numbers n,l and the number j, which determines the total moment, that is, E = E nlj .
The wave functions will depend on the spin variable S z and will be different for different j: .
Quantum levels for a given l, differing in value j, are close to each other (they differ by the energy of the spin-orbit interaction). Four of numbers n, l, j, m j can take the following values:
n= 1, 2, 3,…; l= 0, 1, 2,…, n- 1; j = l+l s or | l - l s |; l s= ±1/2;
-j? m j ? j.
The value of the orbital moment l is denoted in spectroscopy by the letters s, p, d, f, etc. The main quantum number is placed in front of the letters. number at the bottom right j. Therefore, for example, the level (term) with n= 3, l = 1, j= 3/2 are denoted as 3 R 3/2. Figure 21 shows the level diagram of a hydrogen-like atom, taking into account the multiplet structure. Lines 5890 ? and 5896? form

known sodium doublet: yellow lines D2 and D1. 2 s-therm moved far away from 2 R-terms, as it should be in hydrogen-like atoms ( l- degeneracy removed).
Each of the considered levels E nl belongs (2 j+ 1) states that differ in the number m j, that is, the orientation of the total moment J in space. Only when an external field is applied can these merging levels separate. In the absence of such a field, we have (2 j+ 1)-fold degeneracy. So term 2 s 1/2 has degeneracy 2: two states that differ in spin orientation. Therm 2 R 3/2 has fourfold degeneracy according to the orientations of the moment J, m j= ±1/2, ±3/2.
ZEEMAN EFFECT. P. Zeeman, studying the emission spectrum of sodium vapor placed in an external magnetic field, discovered the splitting of spectral lines into several components. Subsequently, on the basis of quantum mechanical concepts, this phenomenon was explained by splitting in a magnetic field energy levels atom.
Electrons in an atom can only be in certain discrete states, when they change, a light quantum is emitted or absorbed. The energy of an atomic level depends on the total orbital momentum, characterized by the orbital quantum number L, and the total spin of its electrons, characterized by the spin quantum number S. Number L can only take integers, S- whole and half integer (in units h). In the direction they can take respectively (2 L+ 1) and (2 S+ 1) positions in space. So the data layer L and S degenerate: it consists of (2 L+ 1)(2S +1) sublevels, the energies of which (if the spin-orbit interaction is ignored) coincide.
The spin-orbit interaction leads, however, to the fact that the level energy depends not only on the quantities L and S, but also from relative position vectors of orbital momentum and spin. Therefore, the energy also depends on the total moment M = M L + M S, determined by the quantum number J, and the level with given L and S splits into several sublevels (forming a multiplet) with different J. This splitting is called the fine level structure. Due to the fine structure, the spectral lines are also split. For example, D- the sodium line corresponds to the transition from the level L = 1 , S= ½ per level c L = 0, S= S. The first of them (levels) is a doublet corresponding to the possible values J= 3/2 and J= Ѕ ( J =L + S; S= ±1/2), while the second one does not have a fine structure. So D-line consists of two very close lines with wavelengths of 5896? and 5890?.
Each level of the multiplet still remains degenerate due to the possibility of orientation of the total mechanical moment in space according to (2 j+ 1) directions. In a magnetic field, this degeneracy is removed. The magnetic moment of an atom interacts with the field, and the energy of such interaction depends on the direction. Therefore, depending on the direction, the atom acquires different additional energies in the magnetic field, and the Zeeman level splits into (2 j+ 1) sublevels.
Distinguish normal (simple) Zeeman effect when each line is split into three components and anomalous (complex) when each line is split into more than three components.
To understand the general patterns of the Zeeman effect, consider simplest atom is a hydrogen atom. If a hydrogen atom is placed in an external uniform magnetic field with induction AT, then due to the interaction of the magnetic moment R m with an external field, the atom will acquire an additional dependence depending on the modules and mutual orientation AT and pm energy
UB= -pmB = -pmBB,
where pmB- projection of the magnetic moment of the electron on the direction of the field.
Given that R mB =-ehm l /(2m)(magnetic quantum number m l= 0, ±1, ±2, …, ±l), we obtain
Bohr magneton.
Total energy of a hydrogen atom in a magnetic field
where the first term is the energy of the Coulomb interaction between an electron and a proton.
It follows from the last formula that in the absence of a magnetic field (B = 0) the energy level is determined only by the first term. When is V? 0, you need to take into account the various allowed values m l . Since for given n and l number m l can take 2 l+ 1 possible values, then the original level will split into 2 l+ 1 sublevels.
On fig. 22a shows possible transitions in the hydrogen atom between states R(l= 1) and s (l= 0). In a magnetic field, the p-state splits into three sublevels (for l = 1, m = 0, ±1), from each of which transitions to the s level can occur, and each transition is characterized by its own frequency: Therefore, a triplet appears in the spectrum (the normal effect Zeeman). Note that the transitions obey the rules for selecting quantum numbers:
On fig. 22b shows the splitting of energy levels and spectral lines for the transition between states d(l= 2) and p(l= 1). State d in a magnetic field

is split into five sublevels, the state p - into three. When taking into account the transition rules, only the transitions indicated in the figure are possible. As can be seen, a triplet appears in the spectrum (the normal Zeeman effect).
The normal Zeeman effect is observed if the original lines do not have a fine structure (they are singlets). If the initial levels have a fine structure, then the spectrum appears more component and the anomalous Zeeman effect is observed.
MECHANICAL AND MAGNETIC MOMENTS OF AN ELECTRON
Orbital magnetic moment of an electron
Each current, as you know, generates a magnetic field. Therefore, an electron whose orbital mechanical moment differs from zero must also have a magnetic moment.
From the classical representations, the angular momentum has the form
where is the velocity and is the radius of curvature of the trajectory.
The magnetic moment of a closed current with an area creates a magnetic moment
is the unit normal to the plane, and are the charge and mass of the electron.
Comparing (3.1) and (3.2), we obtain
The magnetic moment is related to the mechanical moment by the factor
which is called the magnetomechanical (gyromagnetic) ratio for an electron.
For projections of moments, we have the same relationship
The transition to quantum mechanics is carried out by replacing the numerical equations with operator equations
Formulas (3.5) and (3.6) are valid not only for an electron in an atom, but also for any charged particles that have a mechanical moment.
The eigenvalue of the operator is
where is the magnetic quantum number (see Section 2.1)
The constant is called the Bohr magneton
In SI units, it is J/T.
In the same way, one can obtain the eigenvalues of the magnetic moment
where is the orbital quantum number.
Frequently used notation
where . The minus sign is sometimes omitted.
Intrinsic mechanical and magnetic moments of an electron (spin)
The electron has a fourth degree of freedom, which is associated with its own mechanical (and, consequently, magnetic) moment of the electron, the spin. The presence of spin follows from the relativistic Dirac equation
where is a vector matrix and are four-row matrices.
Since the quantities are four-row matrices, the wave function must have four components, which are conveniently written as a column. We will not carry out solutions (3.12), but we will postulate the presence of a spin (intrinsic moment) of an electron, as some empirical requirement, without trying to explain its origin.
Let us dwell briefly on those experimental facts from which the existence of the electron spin follows. One of such direct evidence is the results of the experiment of the German physicists Stern and Gerlach (1922) on spatial quantization. In these experiments, beams of neutral atoms were passed through an area in which an inhomogeneous magnetic field was created (Fig. 3.1). In such a field, a particle with a magnetic moment acquires energy and a force will act on it
which can split the beam into individual components.
In the first experiments, beams of silver atoms were studied. The beam was passed along the axis, and splitting along the axis was observed. The main component of the force is
If the silver atoms are not excited and are at the lower level, that is, in the state (), then the beam should not split at all, since the orbital magnetic moment of such atoms is equal to zero. For excited atoms () the beam would have to split into an odd number of components in accordance with the number of possible values of the magnetic quantum number ().
In fact, the splitting of the beam into two components was observed. This means that the magnetic moment that causes splitting has two projections on the direction of the magnetic field, and the corresponding quantum number takes on two values. The results of the experiment prompted the Dutch physicists Uhlenbeck and Goudsmit (1925) to put forward a hypothesis about the electron has its own mechanical and associated magnetic moments.
By analogy with the orbital number, we introduce the quantum number , which characterizes the intrinsic mechanical moment of the electron. We determine by the number of splits . Hence,
The quantum number is called the spin quantum number, and it characterizes the intrinsic or spin moment of momentum (or simply "spin"). The magnetic quantum number, which determines the projections of the spin mechanical moment and the spin magnetic moment of the spin, has two meanings. Since , and , then no other values exist, and, therefore,
Term spin derived from English word spin, which means to spin.
The spin angular momentum of an electron and its projection are quantized according to the usual rules:
As always, when measuring quantities, one of two possible values \u200b\u200bis obtained. Any superposition of them is possible before measurement.
The existence of spin cannot be explained by the rotation of an electron around its own axis. The maximum value of the mechanical moment can be obtained if the electron mass is distributed along the equator. Then, to obtain the magnitude of the moment of the order line speed points of the equator should be m / s ( m - the classical radius of the electron), that is, much more than the speed of light. Thus, a nonrelativistic consideration of the spin is impossible.
Let us return to the experiments of Stern and Gerlach. Knowing the value of the splitting (in terms of ), one can calculate the value of the projection of the spin magnetic moment on the direction of the magnetic field . It makes up one Bohr magneton.
Let's get the relationship between and :
Value
is called the spin magnetomechanical ratio and is twice the orbital magnetomechanical ratio.
The same relationship exists between the spin magnetic and mechanical moments:
Let's find now the value:
However, it is customary to say that the spin magnetic moment of an electron is equal to one Bohr magneton. This terminology has developed historically and it is connected with the fact that when measuring the magnetic moment, we usually measure its projection, and it is exactly equal to 1.
The electron has its own mechanical angular momentum L s , called spin. Spin is an inherent property of an electron, like its charge and mass. The electron spin corresponds to its own magnetic moment P s , proportional to L s and directed in the opposite direction: P s =g s L s , g s is the gyromagnetic ratio of the spin moments. Projection of intrinsic magnetic moment onto vector B direction: P sB =eh/2m= B , whereh=h/2, B = Bohr magneton. The total magnetic moment of the atom p a = the vector sum of the magnetic moments of the electron entering the atom: P a =p m +p ms . Experience of Stern and Gerlach. By measuring the magnetic moments, they found that a narrow beam of hydrogen atoms in an inhomogeneous magnetic field splits into 2 beams. Although in this state (the atoms were in the S state) the angular momentum of the electron is 0, and the magnetic moment of the atom is also 0, so the magnetic field does not affect the movement of the hydrogen atom, that is, there should be no splitting. However, further studies have shown that the spectral lines of hydrogen atoms show such a structure even in the absence of a magnetic field. Subsequently, it was found that such a structure of spectral lines is explained by the fact that the electron has its own indestructible mechanical moment, called spin.
21. Orbital, spin and total angular and magnetic moment of an electron.
The electron has own moment momentum M S , which is called spin. Its value is determined according to the general laws of quantum mechanics: M S = h= h[(1/2)*(3/2)]=(1/2) h3, M l = h – orbital moment. The projection can take quantum values that differ from each other by h. M Sz =m S h, (m s =S), M lz =m l h. To find the value of the intrinsic magnetic moment, we multiply M s by the ratio of s to M s , s is the intrinsic magnetic moment:
s =-eM s /m e c=-(е h/m e c)=- B 3, B – Bohr magneton.
Sign (-) because M s and s are directed to different sides. The moment of the Electron is composed of 2: orbital M l and spin M s . This addition is carried out according to the same quantum laws, according to which the orbital moments of different electrons are added: Мj= h, j is the quantum number of the total angular momentum.
22. Atom in an external magnetic field. Zeeman effect .
The Zeeman effect is the splitting of energy levels under the action of a magnetic field on atoms. The splitting of the levels leads to the splitting of the spectral lines into several components. The splitting of spectral lines under the action of a magnetic field on radiating atoms is also called the Zeeman effect. The Zeeman splitting of levels is explained by the fact that an atom with a magnetic moment j acquires additional energy in a magnetic field E=- jB B, jB is the projection of the magnetic moment onto the direction of the field. jB =- B gm j , E= B gm j , ( j =0, 1,…, J). The energy level is split into sublevels, and the amount of splitting depends on the quantum numbers L,S,J of the given level.