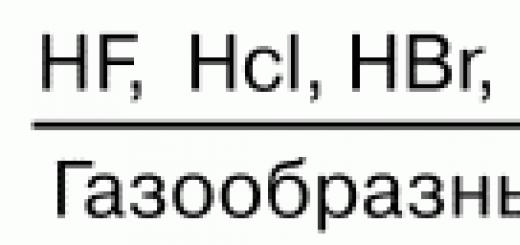How this process was discovered and described. Its use as a source of energy and nuclear weapons is disclosed.
"Indivisible" atom
The twenty-first century is replete with such expressions as "the energy of the atom", "nuclear technology", "radioactive waste". Every now and then in newspaper headlines flickering messages of opportunity radioactive contamination soil, oceans, Antarctic ice. However, an ordinary person often does not have a very good idea of what this field of science is and how it helps in everyday life. It is worth starting, perhaps, with history. From the very first question, which was asked by a well-fed and dressed person, he was interested in how the world works. How the eye sees, why the ear hears, how water differs from stone - this is what worried the wise men from time immemorial. Even in ancient India and Greece, some inquisitive minds suggested that there is a minimal particle (it was also called "indivisible") that has the properties of a material. Medieval chemists confirmed the guess of the sages, and the modern definition of the atom is as follows: an atom is the smallest particle of a substance that is the bearer of its properties.
Parts of an atom
However, the development of technology (in particular, photography) has led to the fact that the atom is no longer considered the smallest possible particle of matter. And although a single atom is electrically neutral, scientists quickly realized that it consists of two parts with different charges. The number of positively charged parts compensates for the number of negative ones, so the atom remains neutral. But there was no unambiguous model of the atom. Since classical physics still dominated during that period, various assumptions were made.
Models of the atom
At first, the model "bun with raisins" was proposed. The positive charge, as it were, filled the entire space of the atom, and negative charges were distributed in it, like raisins in a bun. The famous one determined the following: in the center of the atom there is a very heavy element with a positive charge (nucleus), and much lighter electrons are located around. The mass of the nucleus is hundreds of times heavier than the sum of all the electrons (it makes up 99.9 percent of the mass of the entire atom). Thus, Bohr's planetary model of the atom was born. However, some of its elements contradicted the then accepted classical physics. Therefore, a new, quantum mechanics was developed. With its appearance, the non-classical period of science began.

Atom and radioactivity
From all of the above, it becomes clear that the nucleus is the heavy, positively charged part of the atom, which makes up its bulk. When the positions of electrons in the orbit of an atom were well studied, it was time to understand the nature of the atomic nucleus. The ingenious and unexpectedly discovered radioactivity came to the rescue. It helped to reveal the essence of the heavy central part of the atom, since the source of radioactivity is nuclear fission. At the turn of the nineteenth and twentieth centuries, discoveries rained down one after another. The theoretical solution of one problem necessitated new experiments. The results of the experiments gave rise to theories and hypotheses that needed to be confirmed or refuted. Often the greatest discoveries have come about simply because that is how the formula became easy to calculate (like, for example, Max Planck's quantum). Even at the beginning of the era of photography, scientists knew that uranium salts light up a photosensitive film, but they did not suspect that nuclear fission was the basis of this phenomenon. Therefore, radioactivity was studied in order to understand the nature of nuclear decay. Obviously, the radiation was generated by quantum transitions, but it was not entirely clear which ones. The Curies mined pure radium and polonium, working almost by hand uranium ore to get an answer to this question.

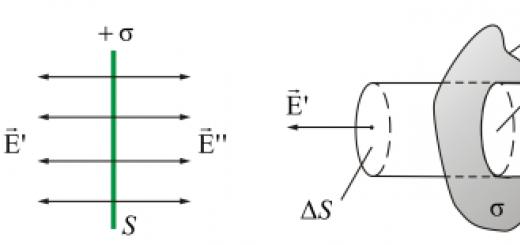
Radiation charge
Rutherford did much to study the structure of the atom and made a contribution to the study of how the fission of the atomic nucleus occurs. The scientist placed the radiation emitted by a radioactive element in a magnetic field and got an amazing result. It turned out that radiation consists of three components: one was neutral, and the other two were positively and negatively charged. The study of nuclear fission began with the determination of its components. It was proved that the nucleus can divide, give up part of its positive charge.
The structure of the nucleus
Later it turned out that the atomic nucleus consists not only of positively charged particles of protons, but also of neutral particles of neutrons. Together they are called nucleons (from the English "nucleus", the nucleus). However, scientists again ran into a problem: the mass of the nucleus (that is, the number of nucleons) did not always correspond to its charge. In hydrogen, the nucleus has a charge of +1, and the mass can be three, and two, and one. Helium next in the periodic table has a nuclear charge of +2, while its nucleus contains from 4 to 6 nucleons. More complex elements can have many more different masses for the same charge. Such variations of atoms are called isotopes. Moreover, some isotopes turned out to be quite stable, while others quickly decayed, since they were characterized by nuclear fission. What principle corresponded to the number of nucleons of the stability of nuclei? Why did the addition of just one neutron to a heavy and quite stable nucleus lead to its splitting, to the release of radioactivity? Oddly enough, the answer to this important question has not yet been found. Empirically, it turned out that stable configurations of atomic nuclei correspond to certain amounts of protons and neutrons. If there are 2, 4, 8, 50 neutrons and/or protons in the nucleus, then the nucleus will definitely be stable. These numbers are even called magic (and adult scientists, nuclear physicists, called them that). Thus, the fission of nuclei depends on their mass, that is, on the number of nucleons included in them.

Drop, shell, crystal
It has not yet been possible to determine the factor responsible for the stability of the core. There are many theories of the model. The three most famous and developed ones often contradict each other on various issues. According to the first, the nucleus is a drop of a special nuclear liquid. Like water, it is characterized by fluidity, surface tension, coalescence and decay. In the shell model, there are also certain energy levels in the nucleus, which are filled with nucleons. The third asserts that the nucleus is a medium that is capable of refracting special waves (de Broglie), while the refractive index is. However, not a single model has yet been able to fully describe why, at a certain critical mass of this particular chemical element, the splitting of the nucleus begins.

What is the decay
Radioactivity, as mentioned above, was found in substances that can be found in nature: uranium, polonium, radium. For example, freshly mined, pure uranium is radioactive. The splitting process in this case will be spontaneous. Without any external influences, a certain number of uranium atoms will emit alpha particles, spontaneously converting into thorium. There is an indicator called the half-life. It shows for what period of time from the initial number of the part about half will remain. Each radioactive element has its own half-life - from fractions of a second for California to hundreds of thousands of years for uranium and cesium. But there is also forced radioactivity. If the nuclei of atoms are bombarded with protons or alpha particles (helium nuclei) with high kinetic energy, they can "split". The mechanism of transformation, of course, is different from how mother's favorite vase is broken. However, there is a certain analogy.

Atom energy
So far, we have not answered a practical question: where does the energy come from during nuclear fission. To begin with, it must be clarified that during the formation of a nucleus, special nuclear forces act, which are called the strong interaction. Since the nucleus is made up of many positive protons, the question remains how they stick together, because the electrostatic forces must push them away from each other quite strongly. The answer is both simple and not at the same time: the nucleus is held together by a very fast exchange between nucleons of special particles - pi-mesons. This connection lives incredibly short. As soon as the exchange of pi-mesons stops, the nucleus decays. It is also known for certain that the mass of a nucleus is less than the sum of all its constituent nucleons. This phenomenon is called the mass defect. In fact, the missing mass is the energy that is expended to maintain the integrity of the nucleus. As soon as some part is separated from the nucleus of an atom, this energy is released and converted into heat in nuclear power plants. That is, the energy of nuclear fission is a clear demonstration of the famous Einstein formula. Recall that the formula says: energy and mass can turn into each other (E=mc 2).
Theory and practice
Now we will tell you how this purely theoretical discovery is used in life to produce gigawatts of electricity. First, it should be noted that controlled reactions use forced nuclear fission. Most often it is uranium or polonium, which is bombarded by fast neutrons. Secondly, it is impossible not to understand that nuclear fission is accompanied by the creation of new neutrons. As a result, the number of neutrons in the reaction zone can increase very quickly. Each neutron collides with new, still intact nuclei, splits them, which leads to an increase in heat release. This is the nuclear fission chain reaction. An uncontrolled increase in the number of neutrons in a reactor can lead to an explosion. This is exactly what happened in 1986 at the Chernobyl nuclear power plant. Therefore, in the reaction zone there is always a substance that absorbs excess neutrons, preventing a catastrophe. It is graphite in the form of long rods. The rate of nuclear fission can be slowed down by immersing the rods in the reaction zone. The equation is drawn up specifically for each active radioactive substance and the particles bombarding it (electrons, protons, alpha particles). However, the final energy output is calculated according to the conservation law: E1+E2=E3+E4. That is, the total energy of the original nucleus and particle (E1 + E2) must be equal to the energy of the resulting nucleus and the energy released in free form (E3 + E4). The nuclear reaction equation also shows what kind of substance is obtained as a result of decay. For example, for uranium U=Th+He, U=Pb+Ne, U=Hg+Mg. The isotopes of chemical elements are not given here, but this is important. For example, there are as many as three possibilities for the fission of uranium, in which different isotopes of lead and neon are formed. In almost one hundred percent of cases, the nuclear fission reaction produces radioactive isotopes. That is, the decay of uranium produces radioactive thorium. Thorium can decay to protactinium, that to actinium, and so on. Both bismuth and titanium can be radioactive in this series. Even hydrogen, which contains two protons in the nucleus (at the rate of one proton), is called differently - deuterium. Water formed with such hydrogen is called heavy water and fills the primary circuit in nuclear reactors.
Non-peaceful atom
Expressions such as "arms race", " cold war”, “nuclear threat” to modern man may seem historical and irrelevant. But once upon a time, every news release almost all over the world was accompanied by reports about how many types of nuclear weapons were invented and how to deal with them. People built underground bunkers and stocked up just in case. nuclear winter. Entire families worked to build the shelter. Even the peaceful use of nuclear fission reactions can lead to disaster. It would seem that Chernobyl taught humanity to be careful in this area, but the elements of the planet turned out to be stronger: the earthquake in Japan damaged the very reliable fortifications of the Fukushima nuclear power plant. The energy of a nuclear reaction is much easier to use for destruction. Technologists only need to limit the force of the explosion, so as not to accidentally destroy the entire planet. The most "humane" bombs, if you can call them that, do not pollute the surroundings with radiation. In general, most often they use an uncontrolled chain reaction. What they strive to avoid at nuclear power plants by all means is achieved in bombs in a very primitive way. For any naturally radioactive element, there is some critical mass pure substance in which the chain reaction starts by itself. For uranium, for example, it is only fifty kilograms. Since uranium is very heavy, it is only a small metal ball 12-15 centimeters in diameter. The first atomic bombs dropped on Hiroshima and Nagasaki were made exactly according to this principle: two unequal parts of pure uranium simply combined and generated a terrifying explosion. Modern weapons are probably more sophisticated. However, one should not forget about the critical mass: there must be barriers between small volumes of pure radioactive material during storage, preventing the parts from connecting.

Sources of radiation
All elements with a nuclear charge greater than 82 are radioactive. Almost all lighter chemical elements have radioactive isotopes. The heavier the nucleus, the shorter its lifetime. Some elements (such as California) can only be obtained artificially - by colliding heavy atoms with lighter particles, most often in accelerators. Since they are very unstable, earth's crust they do not exist: during the formation of the planet, they very quickly disintegrated into other elements. Substances with lighter nuclei, such as uranium, can be mined. This process is long, uranium suitable for extraction, even in very rich ores, contains less than one percent. The third way, perhaps, indicates that a new geological epoch has already begun. This is the extraction of radioactive elements from radioactive waste. After fuel is spent at a power plant, on a submarine or aircraft carrier, a mixture of the original uranium and the final substance, the result of fission, is obtained. At the moment, this is considered solid radioactive waste and there is an acute question of how to bury it so that it does not contaminate environment. However, it is likely that in the near future ready-made concentrated radioactive substances (for example, polonium) will be extracted from these wastes.
November 26, 1894. The wedding of the Russian Tsar Nicholas II and the German princess Alice of Hesse-Darmstadt took place in St. Petersburg. After the wedding, the wife of the emperor adopted the Orthodox faith and received the name Alexandra Feodorovna.
November 27, 1967. The premiere of the first Soviet thriller Viy took place at the Mir cinema in Moscow. The main roles were played by Leonid Kuravlev and Natalia Varley. Filming took place in the Ivano-Frankivsk region and the village of Sednev in the Chernihiv region.
November 28, 1942 Soviet Union concluded an agreement with France on the joint fight against Nazi Germany in the sky. The first French aviation squadron "Normandie-Niemen" consisted of 14 pilots and 17 technical workers.
November 29, 1812 Napoleon's army was defeated while crossing the Berezina River. Napoleon lost about 35 thousand people. The loss of Russian troops, according to the inscription on the 25th wall of the gallery of military glory of the Cathedral of Christ the Savior, amounted to 4 thousand soldiers. Nearly 10,000 Frenchmen were taken prisoner by the Russian General Peter Wittgenstein.
December 1, 1877 Mykola Leontovich, Ukrainian composer, choir conductor, songwriter Dudarik, They Carry a Cossack, Little Mother One Daughter, Shchedryk, was born in the village of Markovka, Vinnytsia region. Bells").
December 1, 1991. An all-Ukrainian referendum on the issue of state independence of Ukraine was held. Leonid Kravchuk was elected the first president of the country.
December 2, 1942. Physicist Enrico Fermi, with a group of American scientists from the University of Chicago, carried out a controlled nuclear reaction, splitting the atom for the first time.
On December 1, 1992, the Ukrainian domain UA was registered in the international database
Among former Soviet republics Ukraine became the first country to receive a national domain on the Internet on December 1, 1992. Russia was registered later: the RU domain appeared on April 7, 1994. In the same year, the Republic of Belarus - BY, Armenia - AM and Kazakhstan - KZ received their domains. And the first national domain in the history of the Internet was the American US, it was registered in March 1985. At the same time, the domains of Great Britain - UK and Israel - IL appeared. The creation of a domain system made it possible to immediately understand by the name of the site where it is located.
In January 1993, at a conference of Ukrainian Internet specialists in the village of Slavske, Lviv region, 27 domains were proposed, created according to a geographical principle, selected by a telephone numbering code. Ukrainian cities and enterprises got the opportunity to create their own websites on the Internet, for example, kiev.ua, crimea.ua, dnepropetrovsk.ua. All responsibilities for their administration were still performed by individuals on a voluntary basis. In some public domains, this practice has been preserved to this day. Now each national or geographic domain has its own administrator - a company or individual who determines the registration rules. Over time, the Internet has spawned its own version of the language. A domain name that ends with the abbreviation COM, NET, EDU means an abbreviation of a general concept. For example, COM - commercial, NET - network, EDU - educational. In our country, the most popular domain is COM. In the spring of 2001, in order to restore order, a legal entity, Hostmaster LLC, was finally created, which included administrators of UA and other Ukrainian domains. Individuals, former owners of the Ukrainian UA domain, officially transferred part of their powers to Hostmaster.
Now everyone can create their own website and get a domain. The first stage, at which only trademark owners could register domains in the UA zone, has already ended. Since 2010, a free domain registration for a period of ten years is available for anyone, the price of using a domain for one year is 90 hryvnias. By the way, the Internet writer, philosopher and public figure XIX century Vladimir Odoevsky. In the novel Year 4338, published in 1837, Odoevsky wrote: Magnetic telegraphs are arranged between familiar houses, through which people living at a distance communicate with each other.". Now, by opening a website on the Internet, without leaving home, each of us can buy an air and railway ticket, make purchases in an electronics supermarket, publish our works without intermediaries, and even find a life partner on a dating site. Twenty-year-olds can hardly imagine an era when they went to the library to get books, letters were written by hand, and news was learned only from television programs or print publications.
6. The world of subatomic particles
Atom splitting
It is often said that there are two kinds of sciences - big sciences and small ones. The splitting of the atom is a big science. It has gigantic experimental facilities, colossal budgets, and receives the lion's share of the Nobel Prizes.
Why did physicists need to split the atom? The simple answer - to understand how the atom works - contains only a fraction of the truth, but there is also a more general reason. To speak literally of the splitting of the atom is not entirely correct. In fact we are talking on the collision of high-energy particles. In the collision of subatomic particles moving at high speeds, a new world of interactions and fields is born. Fragments of matter carrying enormous energy, scattered after collisions, conceal the secrets of nature, which from the "creation of the world" remained buried in the depths of the atom.
Installations on which high-energy particle collisions are carried out - particle accelerators - amaze with their size and cost. They reach several kilometers across, and in comparison with them, even the laboratories in which particle collisions are studied seem tiny. In other areas scientific research the equipment is located in the laboratory; in high-energy physics, laboratories are attached to the accelerator. Recently, the European Center for Nuclear Research (CERN), located near Geneva, allocated several hundred million dollars for the construction of a ring accelerator. The circumference of the tunnel being built for this purpose reaches 27 km. The accelerator, called the LEP (LEP, Large Electron-Positron ring - a large electron-positron ring), is designed to accelerate electrons and their antiparticles (positrons) to speeds that are only a hair's breadth away from the speed of light. To get an idea of the scale of energy, imagine that instead of electrons, a penny coin is accelerated to such speeds. At the end of the acceleration cycle, it would have enough energy to generate $1,000 million worth of electricity! It is not surprising that such experiments are usually classified as "high-energy" physics. Moving towards each other inside the ring, the beams of electrons and positrons experience head-on collisions, in which electrons and positrons annihilate, releasing energy sufficient to create dozens of other particles.
What are these particles? Some of them are the very "bricks" from which we are built: protons and neutrons that make up atomic nuclei, and electrons circulating around the nuclei. Other particles are usually not found in the matter around us: their lifetime is extremely short, and after it expires, they decay into ordinary particles. The number of varieties of such unstable short-lived particles is amazing: several hundred of them are already known. Like stars, unstable particles are too numerous to be distinguished "by name". Many of them are indicated only by Greek letters, and some are simply numbers.
It is important to keep in mind that all these numerous and diverse unstable particles are by no means in the literal sense constituent parts protons, neutrons or electrons. Colliding, high-energy electrons and positrons do not at all scatter into many subatomic fragments. Even in collisions of high-energy protons, which obviously consist of other objects (quarks), they, as a rule, do not split into constituent parts in the usual sense. What happens in such collisions is better seen as the direct production of new particles from the energy of the collision.
About twenty years ago, physicists were completely bewildered by the abundance and variety of new subatomic particles, which seemed to have no end. It was impossible to understand for what so many particles. Maybe the elementary particles are like the inhabitants of the zoo with their implicit belonging to families, but without any clear taxonomy. Or perhaps, as some optimists believed, elementary particles hold the key to the universe? What are the particles observed by physicists: insignificant and random fragments of matter or the outlines of a vaguely perceived order that appear before our eyes, indicating the existence of a rich and complex structure of the subnuclear world? Today there is no doubt about the existence of such a structure. The microcosm has a deep and rational order, and we begin to understand what the meaning of all these particles is.
The first step towards understanding the microcosm was taken as a result of the systematization of all known particles, just as in the 18th century. biologists compiled detailed catalogs of plant and animal species. The most important characteristics of subatomic particles are mass, electric charge, and spin.
Since mass and weight are related, particles with a large mass are often referred to as "heavy". Einstein relation E \u003d mc ^ 2 indicates that the mass of a particle depends on its energy and hence on its speed. A moving particle is heavier than a particle at rest. When people talk about the mass of a particle, they mean it. rest mass, since this mass is independent of the state of motion. A particle with zero rest mass moves at the speed of light. The most obvious example of a particle with zero rest mass is the photon. It is believed that the electron is the lightest of the particles with non-zero rest mass. The proton and neutron are almost 2,000 times heavier, while the mass of the heaviest particle that has been created in the laboratory (Z-particles) is about 200,000 times the mass of an electron.
The electric charge of the particles varies in a rather narrow range, but, as we noted, it is always a multiple of the fundamental unit of charge. Some particles, such as photons and neutrinos, have no electrical charge. If the charge of a positively charged proton is taken as +1, then the charge of an electron is -1.
In ch. 2 we have introduced one more particle characteristic - spin. It also always takes values that are multiples of some fundamental unit, which for historical reasons is chosen to be 1 /2. Thus, the proton, neutron and electron have a spin 1/2, and the photon's spin is 1. Particles with spins 0, 3/2, and 2 are also known. Fundamental particles with spins greater than 2 have not been found, and theorists believe that particles with such spins do not exist.
The spin of a particle is an important characteristic, and depending on its value, all particles are divided into two classes. Particles with spins 0, 1 and 2 are called "bosons" - in honor of the Indian physicist Chatyendranath Bose, and particles with a half-integer spin (i.e. with spin 1/2 or 3/2 - "fermions" in honor of Enrico Fermi. Belonging to one of these two classes is probably the most important in the list of particle characteristics.
Another important characteristic of a particle is its lifetime. Until recently it was believed that electrons, protons, photons and neutrinos are absolutely stable, i.e. have an infinite lifetime. The neutron remains stable as long as it is "locked" in the nucleus, but a free neutron decays in about 15 minutes. All other known particles are highly unstable, their lifetimes vary from a few microseconds to 10-23 s. Such time intervals seem incomprehensibly small, but it should not be forgotten that a particle flying at a speed close to the speed of light (and most of the particles produced in accelerators move at precisely such speeds) manages to fly a distance of 300 m in a microsecond.
Unstable particles undergo decay, which is a quantum process, and therefore there is always an element of unpredictability in decay. The lifespan of a particular particle cannot be predicted in advance. Based on statistical considerations, only the average lifetime can be predicted. One usually speaks of the half-life of a particle, the time it takes for a population of identical particles to be reduced by half. The experiment shows that the decrease in the population occurs exponentially (see Fig. 6) and the half-life is 0.693 of the average lifetime.
It is not enough for physicists to know that this or that particle exists - they strive to understand what its role is. The answer to this question depends on the properties of the particles listed above, as well as on the nature of the forces acting on the particle from outside and inside it. First of all, the properties of a particle are determined by its ability (or inability) to participate in strong interaction. The particles participating in the strong interaction form a special class and are called androns. Particles that participate in the weak interaction and do not participate in the strong interaction are called leptons, which means "lungs". Let's take a brief look at each of these families.
Leptons
The most famous of the leptons is the electron. Like all leptons, it appears to be an elementary point object. As far as is known, the electron has no internal structure; does not consist of any other particles. Although leptons may or may not have an electrical charge, they all have the same spin 1/2, hence they are fermions.
Another well-known lepton, but without a charge, is the neutrino. As already mentioned in Chap. 2, neutrinos are elusive, like ghosts. Since neutrinos do not participate in either strong or electromagnetic interactions, they almost completely ignore matter, penetrating through it as if it were not there at all. The high penetrating power of neutrinos for a long time made it very difficult to experimentally confirm their existence. It wasn't until almost three decades after the neutrino was predicted that they were finally discovered in the laboratory. Physicists had to wait for the creation of nuclear reactors, during which a huge amount of neutrinos are emitted, and only then was it possible to register a head-on collision of one particle with the nucleus and thereby prove that it really exists. Today, it is possible to carry out much more experiments with neutrino beams, which arise during the decay of particles in an accelerator and have the required characteristics. The overwhelming majority of neutrinos "ignore" the target, but from time to time neutrinos still interact with the target, which makes it possible to obtain useful information about the structure of other particles and the nature of the weak interaction. Of course, experiments with neutrinos, unlike experiments with other subatomic particles, do not require the use of special protection. The penetrating power of neutrinos is so great that they are completely harmless and pass through the human body without causing him the slightest harm.
Despite their intangibility, neutrinos hold a special position among other known particles as they are the most abundant particles in the universe, outnumbering electrons and protons by a billion times. The Universe is essentially a sea of neutrinos, in which inclusions in the form of atoms are occasionally found. It is even possible that the total mass of neutrinos exceeds the total mass of stars, and therefore it is neutrinos that make the main contribution to cosmic gravity. According to a group of Soviet researchers, the neutrino has a tiny, but not zero, rest mass (less than one ten-thousandth the mass of an electron); if this is true, then gravitational neutrinos dominate the universe, which in the future may cause its collapse. So, neutrinos, at first glance, the most "harmless" and incorporeal particles, are capable of causing the collapse of the entire universe.
Other leptons include the muon, discovered in 1936 in the products of the interaction of cosmic rays; it turned out to be one of the first known unstable subatomic particles. In all respects, except for stability, the muon resembles an electron: it has the same charge and spin, participates in the same interactions, but has a greater mass. In about two millionths of a second, a muon decays into an electron and two neutrinos. Muons are widely distributed in nature, they account for a significant part of the background cosmic radiation, which is recorded on the Earth's surface by a Geiger counter.
For many years, the electron and muon were the only known charged leptons. Then, in the late 1970s, a third charged lepton was discovered, called the "tau lepton". With a mass of about 3500 electron masses, the tau lepton is obviously a "heavyweight" in the trio of charged leptons, but in all other respects it behaves like an electron and a muon.
This list of known leptons is by no means exhausted. In the 1960s, it was established that there are several types of neutrinos. A neutrino of one type is born together with an electron during the decay of a neutron, and a neutrino of another type - during the birth of a muon. Each type of neutrino is paired with its own charged lepton; hence, there is an "electron neutrino" and a "muon neutrino". In all likelihood, there should also be a neutrino of the third type, which accompanies the birth of a tau lepton. In this case, the total number of neutrino varieties is three, and the total number of leptons is six (Table 1). Of course, each lepton has its own antiparticle; thus the total number of distinct leptons is twelve.
Table 1
Six leptons correspond to charged and neutral modifications (antiparticles are not included in the table). The mass and charge are expressed in units of the mass and charge of the electron, respectively. There is evidence that neutrinos can have a small mass
hadrons
Unlike the handful of known hadron leptons, there are literally hundreds. This alone suggests that hadrons are not elementary particles, but are built from smaller components. All hadrons participate in strong, weak and gravitational interactions, but they occur in two varieties - electrically charged and neutral. Among the hadrons, the neutron and proton are the most well-known and widespread. The remaining hadrons are short-lived and decay either in less than one millionth of a second due to the weak interaction, or much faster (in the order of 10-23 s) due to the strong interaction.
In the 1950s, physicists were extremely puzzled by the abundance and diversity of hadrons. But little by little, particles were classified according to three important characteristics: mass, charge, and spin. Gradually, signs of order began to appear and a clear picture began to emerge. There were hints that symmetries were hidden behind the apparent chaos of the data. A decisive step in unraveling the mystery of hadrons was taken in 1963, when Murray Gell-Mann and George Zweig of the California Institute of Technology proposed the theory of quarks.


Fig.10 Hadrons are built from quarks. The proton (top) is made up of two u-quarks and one d-quark. The lighter pion (bottom) is a meson consisting of one u-quark and one d-antiquark. Other hadrons are all sorts of combinations of quarks.
The basic idea of this theory is very simple. All hadrons are built from smaller particles called quarks. Quarks can bond with each other in one of two ways. possible ways: either in triplets or quark-antiquark pairs. Comparatively heavy particles are made up of three quarks - baryons, which means "heavy particles". The best known baryons are the neutron and the proton. The lighter quark-antiquark pairs form particles called mesons -"intermediate particles". The choice of such a name is explained by the fact that the first discovered mesons occupied an intermediate position in mass between electrons and protons. To account for all the then known hadrons, Gell-Mann and Zweig introduced three different types ("flavors") of quarks, which received rather bizarre names: and(from up- upper), d(from down- lower) and s (from strange- strange). Assuming the possibility of various combinations of flavors, the existence of a large number of hadrons can be explained. For example, a proton is made up of two and- and one d-quark (Fig. 10), and the neutron is made up of two d-quarks and one u-quark.
For the theory proposed by Gell-Mann and Zweig to be valid, it is necessary to assume that quarks carry a fractional electric charge. In other words, they have a charge, the value of which is either 1/3 or 2/3 of the fundamental unit - the electron charge. A combination of two and three quarks can have a total charge equal to zero or one. All quarks have spin 1/2. so they are fermions. The masses of quarks have not been established as accurately as the masses of other particles, since their binding energy in a hadron is comparable to the masses of the quarks themselves. However, the s quark is known to be heavier and- and d quarks.
Inside hadrons, quarks can be in excited states, in many respects similar to the excited states of an atom, but with much higher energies. The excess energy contained in an excited hadron increases its mass so much that, before the creation of the theory of quarks, physicists mistakenly took excited hadrons for completely different particles. It has now been established that many of the seemingly different hadrons are actually only excited states of the same fundamental set of quarks.
As already mentioned in Chap. 5, quarks are held together by a strong interaction. But they also participate in weak interactions. The weak force can change the flavor of a quark. This is how neutron decay occurs. One of the d-quarks in the neutron turns into a u-quark, and the excess charge carries away the electron that is born at the same time. Similarly, by changing the flavor, the weak interaction leads to the decay of other hadrons.
The existence of s-quarks is necessary for the construction of the so-called "strange" particles - heavy hadrons, discovered in the early 1950s. The unusual behavior of these particles, which prompted their name, was that they could not decay due to the strong interaction, although both themselves and their decay products were hadrons. Physicists have puzzled over why, if both mother and daughter particles belong to the family of hadrons, the strong force does not cause them to decay. For some reason, these hadrons "preferred" the much less intense weak interaction. Why? The theory of quarks naturally solved this riddle. The strong force cannot change the flavor of quarks - only the weak force can. And without a change in flavor, accompanied by the transformation of the s-quark into and- or d-quark, decay is impossible.
In table. Figure 2 shows the various possible combinations of three-flavor quarks and their names (usually just a Greek letter). Numerous excited states are not shown. The fact that all known hadrons can be obtained from various combinations of the three basic particles symbolized the main triumph of the theory of quarks. But despite this success, it was only a few years later that direct physical evidence of the existence of quarks was obtained.
These proofs were obtained in 1969 in a series of historical experiments carried out on a large linear accelerator in Stanford (California, USA) - SLAC. The Stanford experimenters reasoned simply. If there really are quarks in the proton, then collisions with these particles inside the proton can be observed. All that is needed is a subnuclear "projectile" that could be directed directly into the bowels of the proton. It is useless to use another hadron for this purpose, since it has the same dimensions as the proton. An ideal projectile could be a lepton, such as an electron. Since the electron does not participate in the strong interaction, it will not "get stuck" in the medium that quarks form. At the same time, an electron can feel the presence of quarks due to their presence electric charge.
table 2 
The three flavors of quarks, u, d, and s, correspond to the charges +2/3, -1/3, and -1/3; they combine in threes to form the eight baryons shown in the table. Quark-antiquark pairs form mesons. (Some combinations such as sss are omitted.)
In the Stanford experiment, the three-kilometer accelerator essentially served as a giant electron "microscope" that made it possible to image the inside of a proton. A conventional electron microscope makes it possible to distinguish details smaller than one millionth of a centimeter in size. The proton, on the other hand, is several tens of millions of times smaller, and it can only be "probed" by electrons accelerated to an energy of 2.1010 eV. At the time of the Stanford experiments, few physicists adhered to the simplified theory of quarks. Most scientists expected that the electrons would be deflected by the electric charges of the protons, but it was assumed that the charge was evenly distributed inside the proton. If this were true, then mainly weak scattering of electrons would occur, i.e. when passing through protons, electrons would not undergo strong deflections. The experiment showed that the scattering pattern differed sharply from the expected one. Everything happened as if some electrons were hitting tiny hard inclusions and bouncing off them at the most incredible angles. Now we know that quarks are such hard inclusions inside protons.
In 1974, a simplified version of the theory of quarks, which by that time had received recognition among theoreticians, received a sensitive blow. Within a few days, two groups of American physicists - one at Stanford led by Burton Richter, the other at Brookhaven National Laboratory led by Samuel Ting - independently announced the discovery of a new hadron, which was called the psi-particle. In itself, the discovery of a new hadron would hardly have been particularly noteworthy, if not for one circumstance: the fact is that in the scheme proposed by the theory of quarks, there was no place for a single new particle. All possible combinations of u, d, and s quarks and their antiquarks have already been "used up". What is a psi-particle made of?
The problem was solved by turning to an idea that had been in the air for some time: there must be a fourth fragrance that no one had ever seen before. The new fragrance already had its own name - charm (charm), or c. It was suggested that a psi-particle is a meson consisting of a c-quark and a c-antiquark (c), i.e. cc. Since antiquarks are carriers of the antiaroma, the charm of the psi-particle is neutralized, and therefore experimental confirmation of the existence of a new flavor (charm) had to wait until it was possible to detect mesons, in which charmed quarks were paired with anti-quarkamps of other flavors. . A whole string of charmed particles is now known. They are all very heavy, so the charm quark is heavier than the strange quark.
The situation described above was repeated in 1977, when the so-called upsilon meson (UPSILON) entered the scene. This time, without much hesitation, the fifth flavor was introduced, called the b-quark (from bottom - bottom, and more often beauty - beauty, or charm). The upsilon meson is a quark-antiquark pair made up of b quarks and therefore has a hidden beauty; but, as in the previous case, a different combination of quarks finally made it possible to discover "beauty".
The relative masses of quarks can be judged at least from the fact that the lightest of the mesons, the pion, consists of pairs and- and d-quarks with antiquarks. The psi meson is about 27 times, and the upsilon meson is at least 75 times heavier than the pion.
The gradual expansion of the list of known flavors occurred in parallel with the increase in the number of leptons; so the obvious question arose whether there would ever be an end. Quarks were introduced in order to simplify the description of the whole variety of hadrons, but even now there is a feeling that the list of particles is again growing too fast.
Since the time of Democritus, the fundamental idea of atomism has been the recognition that, on a sufficiently small scale, there must exist truly elementary particles, the combinations of which make up the matter around us. Atomistics is attractive because indivisible (by definition) fundamental particles must exist in a very limited number. The diversity of nature is due a large number not constituent parts, but their combinations. When it was discovered that there were many different atomic nuclei, the hope disappeared that what we today call atoms corresponded to the ancient Greeks' idea of elementary particles ah substances. And although by tradition we continue to talk about various chemical "elements", it is known that atoms are not elementary at all, but consist of protons, neutrons and electrons. And as soon as the number of quarks turns out to be too large, there is a temptation to assume that they, too, are complex systems consisting of smaller particles.
Although for this reason there is some dissatisfaction with the quark scheme, most physicists consider quarks to be truly elementary particles - pointlike, indivisible and without internal structure. In this respect they resemble peptones, and it has long been suggested that there must be a deep relationship between these two distinct but structurally similar families. The grounds for such a point of view arise from a comparison of the properties of leptons and quarks (Table 3). Leptons can be grouped in pairs by associating each charged lepton with a corresponding neutrino. Quarks can also be grouped in pairs. Tab. 3 is designed in such a way that each cell repeats the structure located directly in front of it. For example, in the second cell, the muon is represented as a "heavy electron", and the charm and strange quarks are represented as heavy variants. and- and d quarks. From the next cell, you can see that the tau lepton is an even heavier "electron", and the b quark is a heavy version of the d quark. For a complete analogy, one more (tau-leptonian) neutrino and a sixth flavor of quarks, which has already received the name of true (truth, t). At the time of writing this book, the experimental evidence for the existence of t quarks was not yet sufficiently convincing, and some physicists doubted that t quarks even existed.
Table 3

Leptons and quarks naturally pair up. as shown in the table. The world around us consists of the first four particles. But the next groups, apparently, repeat the upper one and consist, in the neutrino crown, of extremely unstable particles.
Can there be a fourth, fifth, etc. vapors containing even heavier particles? If so, then the next generation of accelerators will likely give physicists the ability to detect such particles. However, a curious consideration is expressed, from which it follows that other pairs, except for the three named, do not exist. This consideration is based on the number of neutrino types. We will soon learn that at the moment of the Big Bang, which marked the emergence of the Universe, there was an intense birth of neutrinos. A kind of democracy guarantees each kind of particles the same share of energy as the rest; therefore, the more different types of neutrinos, the more energy is contained in the sea of neutrinos that fills outer space. Calculations show that if there are more than three varieties of neutrinos, then the gravity created by all of them would have a strong perturbing effect on the nuclear processes that took place in the first few minutes of the life of the Universe. Therefore, from these indirect considerations follows a very plausible conclusion that the three pairs shown in Table. 3, all quarks and leptons that exist in nature are exhausted.
It is interesting to note that all ordinary matter in the Universe consists of only two lightest leptons (an electron and an electron neutrino) and two lightest quarks ( and and d). If all other leptons and quarks suddenly ceased to exist, then in the world around us, apparently, very little would change.
It is possible that the heavier quarks and leptons play the role of a kind of stand-in for the lightest quarks and leptons. All of them are unstable and quickly disintegrate into particles located in the upper cell. For example, the tau lepton and the muon decay into electrons, while the strange, charmed, and beautiful particles decay rather quickly into either neutrons or protons (in the case of baryons) or leptons (in the case of mesons). The question arises: for what do all these second and third generation particles exist? Why did nature need them?
Particles - carriers of interactions
Six pairs of leptons and quarks, which form the building material of matter, by no means exhaust the list of known particles. Some of them, such as the photon, are not included in the quark scheme. The particles "left overboard" are not the "bricks of the universe", but form a kind of "glue" that does not allow the world to fall apart, i.e. they are associated with four fundamental interactions.
I remember being told as a child that the Moon causes the oceans to rise and fall during the daily tides. It has always been a mystery to me how the ocean knows where the moon is and follows its movement in the sky. When I learned about gravity already at school, my bewilderment only intensified. How does the moon, having overcome a quarter of a million kilometers of empty space, manage to "reach out" to the ocean? The standard answer - the Moon creates a gravitational field in this empty space, the action of which reaches the ocean, setting it in motion - certainly made some sense, but still did not completely satisfy me. After all, we cannot see the gravitational field of the Moon. Maybe that's just what it says? Does this really explain anything? It always seemed to me that the moon must somehow tell the ocean where it is. There has to be some kind of signal exchange going on between the moon and the ocean so that the water knows where to go.
Over time, it turned out that the idea of a force transmitted through space in the form of a signal is not so far from the modern approach to this problem. In order to understand how such a representation arises, it is necessary to consider in more detail the nature force field. As an example, let's take not ocean tides, but a simpler phenomenon: two electrons approach each other, and then, under the influence of electrostatic repulsion, fly apart in different directions. Physicists call this process the scattering problem. Of course, electrons do not literally push each other. They interact at a distance, through the electromagnetic field generated by each electron.

Fig.11. Scattering of two charged particles. The trajectories of the particles are curved as they approach each other due to the action of the electrical repulsion force.
It is not difficult to imagine a picture of the scattering of an electron by an electron. The electrons are initially separated long distance and have little effect on each other. Each electron moves almost in a straight line (Fig. 11). Then, as the repulsive forces come into play, the trajectories of the electrons begin to curve until the particles are as close as possible; after that, the trajectories diverge, and the electrons scatter, again starting to move along rectilinear, but already diverging trajectories. This kind of model is easy to demonstrate in the laboratory using electrically charged balls instead of electrons. And again the question arises: how does the particle "know" where the other particle is, and accordingly changes its motion.
Although the picture of curved electron trajectories is quite illustrative, it is completely unsuitable in a number of respects. The fact is that electrons are quantum particles and their behavior obeys specific laws. quantum physics. First of all, electrons do not move in space along well-defined trajectories. We can still determine in one way or another the starting and ending points of the path - before and after scattering, but the path itself in the interval between the beginning and end of the movement remains unknown and indefinite. In addition, the intuitive idea of a continuous exchange of energy and momentum between the electron and the field, as if accelerating the electron, contradicts the existence of photons. Energy and momentum can be transferred field only in portions, or quanta. A more accurate picture of the perturbation introduced by the field into the motion of an electron can be obtained by assuming that the electron, absorbing a photon of the field, experiences, as it were, a sudden push. Therefore, on quantum level the act of electron-electron scattering can be depicted as shown in Fig. 12. The wavy line connecting the trajectories of two electrons corresponds to a photon emitted by one electron and absorbed by another. Now the scattering act appears as a sudden change in the direction of movement of each electron

Fig.12. Quantum description of the scattering of charged particles. The interaction of particles is due to the exchange of the interaction carrier, or virtual photon (wavy line).
Diagrams of this kind were first used by Richard Feynman to visually represent the various terms of an equation, and initially they had a purely symbolic meaning. But then Feynman diagrams began to be used to schematically depict the interactions of particles. Such pictures, as it were, supplement the physicist's intuition, but they should be interpreted with a certain degree of caution. For example, there is never a sharp break in the trajectory of an electron. Since we only know the initial and final positions of the electrons, we do not know exactly the moment when the photon is exchanged, and which of the particles emits and which absorbs the photon. All these details are hidden by a veil of quantum uncertainty.
Despite this caveat, Feynman diagrams have proven to be an effective means of describing quantum interactions. A photon exchanged between electrons can be seen as a kind of messenger from one of the electrons, telling the other: "I'm here, so get moving!". Of course, all quantum processes are probabilistic in nature, so such an exchange occurs only with a certain probability. It may happen that electrons exchange two or more photons (Fig. 13), although this is less likely.
It is important to be aware that we do not actually see photons scurrying from one electron to another. Interaction carriers are an "internal affair" of two electrons. They exist solely to tell electrons how to move, and although they carry energy and momentum, the corresponding conservation laws of classical physics do not apply to them. Photons in this case can be likened to a ball exchanged on the court by tennis players. Just as a tennis ball determines the behavior of tennis players on a playground, a photon influences the behavior of electrons.
The successful description of the interaction using a carrier particle was accompanied by an extension of the concept of a photon: a photon turns out to be not only a particle of light that we see, but also a ghostly particle, which is "seen" only by charged particles undergoing scattering. Sometimes the photons we observe are called real, and the photons that carry the interaction are virtual, which is reminiscent of their fleeting, almost ghostly existence. The distinction between real and virtual photons is somewhat arbitrary, but nevertheless these concepts have become widespread.
The description of the electromagnetic interaction using the concept of virtual photons - its carriers - in its meaning goes beyond mere illustrations of a quantum nature. In fact, we are talking about a theory thought out to the smallest detail and equipped with a perfect mathematical apparatus, known as quantum electrodynamics, abbreviated QED. When QED was first formulated (this happened shortly after the Second World War), physicists had at their disposal a theory that satisfies the basic principles of both quantum theory and relativity. This is a great opportunity to see the joint manifestations of two important aspects of the new physics and. test them experimentally.
Theoretically, the creation of QED was an outstanding achievement. Earlier studies of the interaction of photons and electrons had very limited success due to mathematical difficulties. But as soon as theorists learned how to correctly calculate, everything else fell into place. QED proposed a procedure for obtaining the results of any arbitrarily complex process involving photons and electrons.

Fig.13. The scattering of electrons is due to the exchange of two virtual photons. Such processes constitute a small correction to the main process depicted in Fig. eleven
To test how well the theory agrees with reality, physicists focused on two effects of particular interest. The first concerned the energy levels of the hydrogen atom, the simplest atom. QED predicted that the levels should be slightly shifted from the position they would occupy if there were no virtual photons. The theory was very accurate in predicting the magnitude of this shift. An experiment to detect and measure displacement with extreme accuracy was carried out by Willis Lamb of the University of pc. Arizona. To everyone's delight, the results of the calculations perfectly matched the experimental data.
The second decisive test of QED concerned an extremely small correction to the electron's own magnetic moment. And again, the results of theoretical calculations and experiment completely coincided. Theorists began to refine the calculations, the experimenters - to improve the instruments. But, although the accuracy of both theoretical predictions and experimental results was continuously improved, the agreement between QED and experiment remained impeccable. At present, the theoretical and experimental results are still consistent within the achieved accuracy, which means a match of more than nine decimal places. Such a striking correspondence gives the right to consider QED as the most perfect of the existing natural science theories.
Needless to say, after a similar triumph, QED was adopted as a model for the quantum description of the other three fundamental interactions. Of course, the fields associated with other interactions must correspond to other carrier particles. To describe gravity was introduced graviton, playing the same role as a photon. During the gravitational interaction of two particles, an exchange of gravitons occurs between them. This interaction can be visualized using diagrams similar to those shown in Fig. 12 and 13. It is the gravitons that carry signals from the Moon to the oceans, following which they rise at high tide and fall at low tide. Gravitons scurrying between the Earth and the Sun keep our planet in orbit. Gravitons firmly tie us to the Earth.
Like photons, gravitons move at the speed of light, therefore, gravitons are particles with "zero rest mass". But this is where the similarities between gravitons and photons end. While a photon has a spin of 1, a graviton has a spin of 2.
Table 4 
Particles-carriers of four fundamental interactions. The mass is expressed in units of proton mass.
This is an important distinction, because it determines the direction of the force: in electromagnetic interaction, like-charged particles, such as electrons, repel each other, and in gravitational interaction, all particles are attracted to each other.
Gravitons can be real and virtual. A real graviton is nothing but a quantum of a gravitational wave, just as a real photon is a quantum electromagnetic wave. In principle, real gravitons can be "observed". But because the gravitational interaction is incredibly weak, gravitons cannot be detected directly. The interaction of gravitons with other quantum particles is so weak that the probability of scattering or absorption of a graviton, for example, by a proton, is infinitesimal.
The basic idea of the exchange of carrier particles extends to other interactions (Table 4) - weak and strong. However, there are important differences in the details. Recall that the strong interaction ensures the bond between quarks. Such a connection can be created by a force field similar to electromagnetic, but more complex. Electric forces lead to the formation of a bound state of two particles with charges of opposite signs. In the case of quarks, bound states of three particles arise, which indicates a more complex nature of the force field, which corresponds to three types of "charge". Particles - carriers of interaction between quarks, linking them in pairs or triplets, are called gluons.
In the case of weak interaction, the situation is somewhat different. The radius of this interaction is extremely small. Therefore, the carriers of the weak interaction must be particles with large rest masses. The energy contained in such a mass has to be "borrowed" in accordance with the Heisenberg uncertainty principle, which was already discussed on p. 50. But since the "borrowed" mass (and hence the energy) is so large, the uncertainty principle requires that the maturity of such a loan be extremely short - only about 10^-28s. Such short-lived particles do not have time to move very far, and the radius of interaction carried by them is very small.
There are actually two types of weak interaction carriers. One of them is like a photon in everything except the rest mass. These particles are called Z-particles. In essence, Z-particles are a new kind of light. Another type of weak interaction carriers, W-particles, differ from Z-particles by the presence of an electric charge. In ch. 7 we discuss in more detail the properties of the Z- and W-particles, which were discovered only in 1983.
The classification of particles into quarks, leptons, and force carriers completes the list of known subatomic particles. Each of these particles plays its own, but decisive role in the formation of the Universe. If there were no carrier particles, there would be no interactions, and each particle would remain ignorant of its partners. Complex systems could not arise, any activity would be impossible. Without quarks, there would be no atomic nuclei or sunlight. Without leptons, atoms could not exist, chemical structures and life itself would not have arisen.
What are the tasks of elementary particle physics?
The influential British newspaper The Guardian once published an editorial questioning the wisdom of developing particle physics, a costly undertaking that consumes not only a significant share of the national science budget, but also the lion's share of the best minds. “Do physicists know what they are doing?” the Guardian asked. “If they do, what good is it? Who, besides physicists, needs all these particles?”
A few months after this publication, I had the opportunity to attend a lecture in Baltimore by George Keworth, US Presidential Science Adviser. Keyworth also turned to particle physics, but his lecture was delivered in a completely different tone. American physicists were impressed by the recent announcement from CERN, the leading European laboratory for elementary particle physics, about the discovery of fundamental W- and Z-particles, which were finally obtained at the large proton-antiproton colliding beam accelerator (collider). Americans are used to the fact that all sensational discoveries are made in their laboratories of high energy physics. Is not the fact that they gave way to the palm, a sign of scientific and even national decline?
Keworth had no doubt that for the prosperity of the United States in general and the American economy in particular, it is necessary that the country occupies the forefront in scientific research. Key fundamental research projects, Keyworth said, are at the forefront of progress. The United States must regain its dominance in particle physics,
In the same week, information channels circulated about the American project of a giant accelerator designed to conduct a new generation of experiments in elementary particle physics. The main cost was $2 billion, making this accelerator the most expensive machine ever built by man. This giant of Uncle Sam, in comparison with which even the new CERN power line accelerator will seem like a dwarf, is so large that the entire state of Luxembourg could fit inside its ring! Giant superconducting magnets are designed to create intense magnetic fields that will wrap the particle beam along the annular chamber; it is such a huge structure that the new accelerator is supposed to be placed in the desert. I would like to know what the editor of The Guardian thinks about this.
Known as the Superconducting Super Collider (SSC), but more commonly referred to as the "dezertron" (from the English. desert- desert. - Ed.), this monstrous machine will be able to accelerate protons to energies approximately 20 thousand times greater than the rest energy (mass). These figures can be interpreted in different ways. At maximum acceleration, the particles will move at a speed of only 1 km / h less than the speed of light - the limiting speed in the universe. The relativistic effects are so strong that the mass of each particle is 20 thousand times greater than at rest. In the frame associated with such a particle, time is stretched so much that 1 s corresponds to 5.5 hours in our frame of reference. Each kilometer of the chamber through which the particle passes will "seem" to be compressed to only 5.0 cm.
What is the dire need that drives states to expend such huge resources on ever more destructive fission of the atom? Is there any practical use in such research?
Any great science, of course, is not alien to the spirit of struggle for national priority. Here, just as in art or sports, it is pleasant to win prizes and world recognition. Particle physics has become a kind of symbol of state power. If it develops successfully and yields tangible results, then this indicates that science, technology, as well as the country's economy as a whole, are basically at the proper level. This maintains confidence in the high quality of products from other more general technology industries. To create an accelerator and all related equipment requires a very high level of professionalism. The valuable experience gained in the development of new technologies can have an unexpected and beneficial effect on other areas of scientific research. For example, research and development on the superconducting magnets needed for the Desertron has been going on in the US for twenty years. However, they do not provide direct benefits and are therefore difficult to evaluate. Are there any more tangible results?
Another argument is sometimes heard in support of fundamental research. Physics is generally ahead of technology by about fifty years. The practical application of one or the other scientific discovery at first it is by no means obvious, but only a few of the significant achievements of fundamental physics have not found practical applications over time. Recall Maxwell's theory of electromagnetism: could its creator have foreseen the creation and success of modern telecommunications and electronics? And Rutherford's words that nuclear energy is unlikely to ever find practical use? Is it possible to predict what the development of elementary particle physics can lead to, what new forces and new principles will be discovered that will expand our understanding of the world around us and give us power over a wider range of physical phenomena. And this can lead to the development of technologies no less revolutionary in nature than radio or nuclear energy.
Most branches of science eventually found some military application. In this regard, elementary particle physics (unlike nuclear physics) has so far remained untouched. Coincidentally, Keyworth's lecture coincided with the hype surrounding President Reagan's controversial anti-missile project, the so-called beam, weapons (this project is part of a program called the Strategic Defense Initiative, SDI). The essence of this project is to use high-energy particle beams against enemy missiles. This application of particle physics is truly sinister.
The prevailing opinion is that the creation of such devices is not feasible. The majority of scientists working in the field of elementary particle physics consider these ideas absurd and unnatural, and strongly oppose the president's proposal. After condemning the scientists, Keyworth urged them to "think about what role they can play" in the beam weapons project. This appeal by Keyworth to physicists (purely coincidental, of course) followed his words regarding the funding of high energy physics.
It is my firm belief that high-energy physicists do not need to justify the need for fundamental research by referring to applications (especially military ones), historical analogies, or vague promises of possible technical miracles. Physicists conduct these studies primarily in the name of their indestructible desire to find out how our world works, the desire to understand nature in more detail. Particle physics is unparalleled among other human activities. For two and a half millennia, humanity has sought to find the original "bricks" of the universe, and now we are close to the final goal. Giant installations will help us penetrate into the very heart of matter and wrest from nature its innermost secrets. Mankind can expect unexpected applications of new discoveries, previously unknown technologies, but it may turn out that high-energy physics will not give anything for practice. But after all, there is little practical use from a majestic cathedral or concert hall. In this regard, one cannot fail to recall the words of Faraday, who once remarked: "What is the use of a newborn?" The types of human activity far from practice, which include elementary particle physics, serve as evidence of the manifestation of the human spirit, without which we would be doomed in our overly material and pragmatic world.
Choose the appropriate isotope. Some elements or isotopes undergo radioactive decay, and different isotopes may behave differently. The most common isotope of uranium has an atomic weight of 238 and consists of 92 protons and 146 neutrons, but its nuclei usually absorb neutrons without splitting into nuclei of lighter elements. The isotope of uranium, whose nucleus contains three fewer neutrons, 235 U, fissions much more easily than 238 U, and is called a fissile isotope.
- The fission of uranium releases three neutrons that collide with other uranium atoms, resulting in a chain reaction.
- Some isotopes fission so easily and quickly that it is impossible to maintain a constant nuclear reaction. This phenomenon is called spontaneous, or spontaneous, decay. For example, the plutonium isotope 240 Pu is subject to such decay, in contrast to 239 Pu with a lower fission rate.
In order for the reaction to continue after the decay of the first atom, enough isotope must be collected. To do this, it is necessary to have a certain minimum amount of fissile isotope that will support the reaction. This quantity is called the critical mass. Enough starting material is required to reach critical mass and increase the probability of decay.
Shoot one atomic nucleus of an isotope at another nucleus of the same isotope. Since subatomic particles are quite rare in free form, it is often necessary to separate them from the atoms containing these particles. One way to do this is to shoot one atom of the isotope at another atom.
- This method has been used to create atomic bomb from 235 U, which was dropped on Hiroshima. A cannon-like weapon with a uranium core fired 235 U atoms at a target of identical 235 U atoms. The atoms traveled fast enough that the neutrons released from them penetrated the nuclei of other 235 U atoms and split them. The fission, in turn, released neutrons, which split the next 235 U atoms.
Fire at the nuclei of the fissile isotope with subatomic particles. A single subatomic particle can hit a 235 U atom and split it into two separate atoms of other elements, producing three neutrons. Subatomic particles can be obtained from a controlled source (such as a neutron gun) or created from nuclear collisions. Three types of subatomic particles are commonly used.
- Protons. These subatomic particles have mass and a positive electrical charge. The number of protons in an atom determines which element it is an atom of.
- Neutrons. The mass of these subatomic particles is equal to the mass of a proton, but they are neutral (have no electrical charge).
- Alpha particles. These particles are electron-free nuclei of helium atoms. They consist of two protons and two neutrons.
Nuclear fission
The discovery of isotopes of stable elements, the refinement of measurements of the elementary charge were the first achievements of post-war physics (1917-1918). In 1919 a new sensational discovery- artificial fission of the nucleus. This discovery was made by Rutherford in Cambridge at the Cavendish Laboratory, which he headed in the same year, 1919.
Rutherford studied the collision of a-particles with light atoms. Collisions of an a-particle with the nuclei of such atoms should accelerate them. So, when an a-particle hits a hydrogen nucleus, it increases its speed by 1.6 times, and the nucleus takes 64% of its energy from the a-particle. Such accelerated nuclei are easily detected by the scintillations that occur when they hit a zinc sulfide screen. They were actually observed by Marsden in 1914.
Rutherford continued Marsden's experiments, but, as he himself noted, these experiments were "carried out at very irregular intervals, since everyday occupations and work connected with the war allowed ..." "Experiments were even completely stopped for a long time." Only after the end of the war were experiments carried out regularly, and their results were published in 1919 in four articles under the general title "Collisions of a-particles with light atoms."
The instrument used by Rutherford to study such collisions was a brass chamber 18 cm long, 6 cm high, and 2 cm wide. A metal disk coated with an active substance was the source of a-particles. The disc was placed inside the chamber and could be set at different distances from the zinc sulfide screen, on which scintillations were observed using a microscope.
The chamber could be filled with various gases (see Fig. 78).
Rice. 78. Dempester mass spectrograph
When dry oxygen or carbon dioxide was introduced, the number of scintillations decreased due to the absorption of a-particles by the gas layer. “An unexpected effect, however,” Rutherford wrote in the fourth article, “was discovered when dry air was introduced into the apparatus. Instead of decreasing, the number of scintillations increased, and for an absorption corresponding to approximately 19 cm of air, their number was approximately 2 times greater than that observed in vacuum. From this experiment it was clear that a-particles, when passing through air, give rise to scintillations corresponding to large path lengths, the brightness of which for the eye appeared to be approximately equal to the brightness of H-scintillations. Since in oxygen and carbon dioxide Since such an effect was not observed, it could be argued with a high probability that this effect owes its origin to nitrogen.
The chamber was filled with clean, thoroughly dried nitrogen. "In pure nitrogen, the number of scintillations corresponding to a long range was greater than in air." Thus, "the long range scintillations observed in air must be attributed to nitrogen."
It was necessary, however, to show that the long-range a-particles causing scintillations "are the results of collisions of a-particles with nitrogen atoms."

Scheme of the first installation of Millikan
Through numerous experiments, Rutherford showed that this is indeed the case and that as a result of such collisions particles are obtained with a maximum range of 28 cm, the same as that of H atoms. “From the results obtained so far,” wrote Rutherford, “it is difficult to avoid the conclusion that the long-range atoms arising from the collision of a-particles with nitrogen are not nitrogen atoms, but, in all probability, hydrogen atoms or atoms with a mass of 2 If this is so, then we must conclude that the nitrogen atom is disintegrating due to the enormous forces developing in collision with the fast a-particle, and that the liberated hydrogen atom forms a constituent part of the atom.
Thus, the phenomenon of the splitting of nitrogen nuclei during the impact of fast a-particles was discovered, and for the first time the idea was expressed that hydrogen nuclei are an integral part of the nuclei of atoms. Subsequently, Rutherford proposed the term "proton" for this component of the nucleus. Rutherford ended his article with the words: "The results generally indicate that if a-particles or similar fast moving particles with much higher energy could be used for experiments, then destruction of the nuclear structures of many light atoms could be detected."
On June 3, 1920, Rutherford gave the so-called Bakerian Lecture entitled "The Nuclear Structure of the Atom." Reporting in this lecture on the results of his research on the collision of a-particles with atomic nuclei and on the splitting of nitrogen nuclei, Rutherford, discussing the nature of the fission products, made an assumption about the possibility of the existence of nuclei with a mass of 3 and 2 and nuclei with a mass of a hydrogen nucleus, but with zero charge. At the same time, he proceeded from the hypothesis, first expressed by Maria Sklodowska-Curie, that electrons are part of the atomic nucleus.
Rutherford writes that “it seems very plausible to him that one electron can bind two H-nuclei and perhaps even one H-nucleus. If the first assumption is true, then it indicates the possibility of the existence of an atom with a mass of about 2 and with one charge. Such a substance should be considered as an isotope of hydrogen. The second assumption includes the idea of the possibility of the existence of an atom with a mass of 1 and a nuclear charge equal to zero. Such formations seem quite possible... Such an atom would have absolutely fantastic properties. Its external field should practically be equal to zero, with the exception of regions very close to the nucleus; as a result, it should have the ability to pass freely through matter. The existence of such an atom would probably be difficult to detect with a spectroscope and could not be kept in a closed vessel. On the other hand, it should easily enter the structure of the atom and either combine with its nucleus, or be accelerated by the intense field of the latter, giving rise to a charged H-atom or an electron, or both.
This is how the hypothesis about the existence of the neutron and the heavy isotope of hydrogen was put forward. It was expressed on the basis of the hypothesis proposed by M. Sklodowska-Curie that the nuclei of atoms consist of hydrogen nuclei (protons) and electrons.
This notion immediately explained the characteristic nuclear numbers A and Z.
However, such characteristics of the nucleus as mass number A and charge Z turned out to be insufficient. Back in 1924, before the discovery of the spin, W. Pauli suggested that the nucleus has a magnetic moment that affects the motion of orbital electrons and thereby creates a hyperfine structure of spectral lines. The explanation of the fine structure of the spectra by the presence of spin-induced magnetic moments of nuclei led to the division of nuclei into two types. Even-type nuclei with integer spin obey Bose statistics, odd-type nuclei with half-integer spin obey Fermi-Dirac statistics. Therefore, according to the proton-electron theory, nuclei consisting of an even number of electrons and protons must obey Bose statistics, from an odd number - Fermi-Dirac statistics.
In 1930, it turned out that the nitrogen nucleus obeys Bose statistics, although, according to the proton-electron theory of the structure of the nucleus, it consists of 21 particles (14 protons, 7 electrons). This fact is known in science as the nitrogen catastrophe.
In the same year, when the nitrogen catastrophe was discovered, the results of experiments by L. Meitner and Ortman were published, confirming the results of the experiments of Ellis and Wooster in 1927. These experiments showed that the total energy (3-rays, measured by a thick-walled microcalorimeter, is less than the difference between the energies of the initial and finite nuclei, i.e., part of the energy emitted by the nucleus during p-decay disappears, resulting in a flagrant contradiction with the law of conservation of energy.
The solution to the problem of the nitrogen catastrophe and the riddle of p-spectra was given on the basis of the idea of the existence in nature of neutral particles - heavy, called the neutron, and light - called the neutrino, that is, the small neutron, at the suggestion of Fermi.
From The Adventures of Mr. Tompkins the author Gamov GeorgyCHAPTER 12 Inside the Nucleus The next lecture Mr. Tompkins attended was on the interior of the nucleus as the center around which the atomic electrons revolve. "Ladies and gentlemen," began the professor. - More and more delving into the structure of matter, we will try to
From the book [lecture for schoolchildren] author Ivanov Igor Pierovichamazing world inside the atomic nucleus
From the book The Newest Book of Facts. Volume 3 [Physics, chemistry and technology. History and archeology. Miscellaneous] author Kondrashov Anatoly PavlovichThe amazing world inside the atomic nucleus
From the book Neutrino - the ghostly particle of the atom author Asimov Isaac From the book History of Physics Course author Stepanovich Kudryavtsev Pavel From the book Interplanetary Travel [Flights to world space and achievement celestial bodies] author Perelman Yakov IsidorovichThe structure of the nucleus Although the question of the radiation of a?-particle seemed to be finally clarified, since the law of conservation of electric charge was fulfilled, physicists continued their research. It remained a mystery to them how a positively charged nucleus could emit
From the book History of the Atomic Bomb author Mania HubertRepulsion within the nucleus By 1932 it had become clear that nuclei were composed exclusively of protons and neutrons. From more early theories, who claimed that electrons were in the nucleus, refused. Although this solved many problems at once, a question arose that was not there before. Until now
From the book Asteroid-Comet Hazard: Yesterday, Today, Tomorrow author Shustov Boris MikhailovichAttraction inside the nucleus If, when considering atomic nuclei, gravitational interactions are neglected and only electromagnetic interactions are taken into account, it is difficult to explain the existence of the nucleus. The particles of which it is composed could not combine due to colossal forces
From the book of Marie Curie. Radioactivity and the elements [Matter's best kept secret] author Paez Adela MunozThe discovery of the atomic nucleus Let us consider in more detail one of Rutherford's fundamental discoveries - the discovery of the atomic nucleus and the planetary model of the atom. We have seen that the assimilation of the atom to the planetary system was done at the very beginning of the 20th century. But this model was hard
From the author's bookProton-Neutron Model of the Nucleus On May 28, 1932, the Soviet physicist D. D. Ivanenko published a note in Nature in which he suggested that the neutron, along with the proton, is a structural element of the nucleus. He pointed out that such a hypothesis solves the problem of the nitrogen catastrophe. AT
From the author's bookInside the core This unprecedented journey for the passengers of the Jules Verne core will not be as peaceful and safe as it is described in the novel. Do not think, however, that danger threatens them during the journey from the Earth to the Moon. Not at all! If they managed to stay alive by the moment,
From the author's bookTo Chapter VIII 6. Pressure inside a Cannonball For readers who would like to verify the calculations mentioned on page 65, we present here these simple calculations. For calculations, we will have to use only two formulas for accelerated motion, namely: end
From the author's book From the author's book4.2. Physical characteristics, structure of the nucleus B last decade our knowledge of comets and the processes that take place on them has expanded considerably. A sharp increase in interest in comets was facilitated by the preparation and holding of an international space
From the author's bookRutherford and the Discovery of the Atomic Nucleus What happened to someone who, in his youth, was a good rugby player, and then guessed before anyone else that the atom could decay? Ernest Rutherford completed his American "exile" in January 1907, some time after his death.