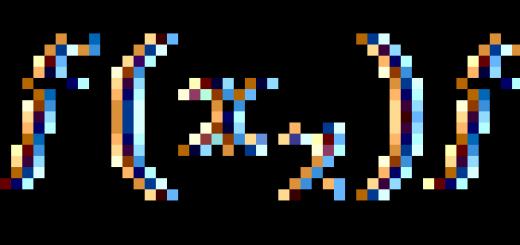Modern symbols chemical elements were introduced into science in 1813 by Berzelius. At his suggestion, the elements are denoted by the initial letters of their Latin names. For example, oxygen (Oxygenium) is denoted by the letter O, sulfur by the letter S, hydrogen (Hydrogenium) by the letter H. In cases where the names of several elements begin with the same letter, one of the following is added to the first letter. So, carbon (Carboneum) has the symbol C, calcium, copper, etc.
Chemical symbols are not only abbreviated names of elements: they also express their certain quantities (or masses), i.e. each symbol denotes either one atom of an element, or one mole of its atoms, or the mass of an element equal to (or proportional to) the molar mass of this element. For example, C means either one carbon atom, or one mole of carbon atoms, or 12 mass units (usually ) of carbon.
The formulas of substances also indicate not only the composition of the substance, but also its quantity and mass. Each formula represents either one molecule of a substance, or one mole of a substance, or the mass of a substance equal to (or proportional to) its molar mass. For example, it denotes either one molecule of water, or one mole of water, or 18 mass units (usually) of water.
Simple substances are also denoted by formulas showing how many atoms a molecule consists of. a simple substance: for example, the formula for hydrogen. If the atomic composition of a molecule of a simple substance is not exactly known or the substance consists of molecules containing a different number of atoms, and also if it has not a molecular, but an atomic or metallic structure, a simple substance is denoted by the element symbol.
For example, a simple substance phosphorus is denoted by the formula P, since, depending on the conditions, phosphorus can consist of molecules with a different number of atoms or have a polymeric structure.
The formula of a substance is established on the basis of the results of its analysis. For example, according to the analysis, glucose contains (wt.) carbon, (wt.) hydrogen and (wt.) oxygen. Therefore, the masses of carbon, hydrogen and oxygen are related to each other as . Let's denote the desired glucose formula , where are the numbers of carbon, hydrogen and oxygen atoms in the molecule. The masses of the atoms of these elements are respectively equal. Therefore, the glucose molecule contains carbon, hydrogen and oxygen. The ratio of these masses is . But we have already found this ratio, based on the data of glucose analysis. Hence:
According to proportion properties:
![]()
Therefore, in a glucose molecule, there are two hydrogen atoms and one oxygen atom per carbon atom. This condition is satisfied by formulas, etc. The first of these formulas - - is called the simplest or empirical formula; it corresponds to a molecular weight of 30.02. In order to find out the true or molecular formula, it is necessary to know the molecular weight of a given substance. When heated, glucose is destroyed without turning into a gas. But its molecular weight can be determined by the methods described in Chapter VII: it is equal to 180. From a comparison of this molecular weight with the molecular weight corresponding to the simplest formula, it is clear that the formula corresponds to glucose.
Having become acquainted with the derivation of chemical formulas, it is easy to understand how the exact values \u200b\u200bof molecular weights are established. As already mentioned, the existing methods for determining molecular weights in most cases do not give quite accurate results. But, knowing at least approximately the molecular weight and percentage composition of a substance, it is possible to establish its formula, expressing the atomic composition of the molecule. Since the molecular weight is equal to the sum of the atomic masses of the atoms that form it, then, by adding the atomic masses of the atoms that make up the molecule, we determine the molecular weight of the substance. The accuracy of the found molecular weight will correspond to the accuracy with which the analysis of the substance was performed.
The decision on the need to maintain such a notebook did not come immediately, but gradually, with the accumulation of work experience.
At first it was a place at the end of the workbook - a few pages for writing down the most important definitions. Then the most important tables were placed there. Then came the realization that in order to learn how to solve problems, most students need strict algorithmic prescriptions, which they, first of all, must understand and remember.
It was then that the decision came to maintain, in addition to the workbook, another obligatory chemistry notebook - a chemical dictionary. Unlike workbooks, which can even be two during one academic year, the dictionary is a single notebook for the entire chemistry course. It is best if this notebook has 48 sheets and a strong cover.
We arrange the material in this notebook as follows: at the beginning - the most important definitions that the guys write out from the textbook or write down under the dictation of the teacher. For example, in the first lesson in the 8th grade, this is the definition of the subject “chemistry”, the concept of “chemical reactions”. During the school year in the 8th grade, they accumulate more than thirty. According to these definitions, I conduct surveys in some lessons. For example, an oral question in a chain, when one student asks a question to another, if he answered correctly, then he already asks the next question; or, when one student is asked questions by other students, if he does not cope with the answer, then they answer themselves. In organic chemistry, these are mainly definitions of classes of organic substances and main concepts, for example, “homologues”, “isomers”, etc.
At the end of our reference book, material is presented in the form of tables and diagrams. On the last page is the very first table “Chemical elements. Chemical signs". Then the tables “Valence”, “Acids”, “Indicators”, “Electrochemical series of voltages of metals”, “Series of electronegativity”.
I especially want to dwell on the contents of the table “Correspondence of acids to acid oxides”:
| Correspondence of acids to acid oxides | ||||
| acid oxide | Acid | |||
| Name | Formula | Name | Formula | Acid residue, valency |
| carbon monoxide (II) | CO2 | coal | H2CO3 | CO 3 (II) |
| sulfur(IV) oxide | SO2 | sulphurous | H2SO3 | SO3(II) |
| sulfur(VI) oxide | SO 3 | sulfuric | H2SO4 | SO4(II) |
| silicon(IV) oxide | SiO2 | silicon | H2SiO3 | SiO 3 (II) |
| nitric oxide (V) | N 2 O 5 | nitric | HNO3 | NO 3 (I) |
| phosphorus(V) oxide | P2O5 | phosphoric | H3PO4 | PO 4 (III) |
Without understanding and memorizing this table, it is difficult for students of the 8th grade to compile reaction equations acid oxides with alkalis.
When studying the theory of electrolytic dissociation, at the end of the notebook we write down schemes and rules.
Rules for compiling ionic equations:
1. In the form of ions, write down the formulas of strong electrolytes that are soluble in water.
2. In molecular form, write down the formulas of simple substances, oxides, weak electrolytes and all insoluble substances.
3. The formulas of poorly soluble substances on the left side of the equation are written in ionic form, on the right - in molecular form.
When studying organic chemistry, we write in the dictionary summarizing tables for hydrocarbons, classes of oxygen- and nitrogen-containing substances, schemes for genetic relationships.
| Physical quantities | |||
| Designation | Name | Units | Formulas |
| amount of substance | mole | = N / N A ; = m / M; V / V m (for gases) |
|
| N A | Avogadro's constant | molecules, atoms and other particles | N A = 6.02 10 23 |
| N | number of particles | molecules, atoms and other particles |
N = N A |
| M | molar mass | g/mol, kg/kmol | M = m / ; / M/ = M r |
| m | weight | g, kg | m = M ; m = V |
| Vm | molar volume of gas | l / mol, m 3 / kmol | Vm \u003d 22.4 l / mol \u003d 22.4 m 3 / kmol |
| V | volume | l, m 3 | V = V m (for gases) ; |
| density | g/ml; | = m/V; M / V m (for gases) |
|
During the 25 years of teaching chemistry at school, I had to work on different programs and textbooks. At the same time, it was always surprising that practically no textbook teaches how to solve problems. At the beginning of the study of chemistry, in order to systematize and consolidate knowledge in the dictionary, the students and I compile a table “Physical quantities” with new quantities:
When teaching students how to solve computational problems, great importance I give algorithms. I believe that the strict prescription of the sequence of actions allows a weak student to understand the solution of problems of a certain type. For strong students, this is an opportunity to reach the creative level of their further chemical education and self-education, since first you need to confidently master a relatively small number of standard techniques. On the basis of this, the ability to correctly apply them at different stages of solving more complex problems will develop. Therefore, I have compiled algorithms for solving computational problems for all types of school course problems and for extracurricular activities.
I will give examples of some of them.
Algorithm for solving problems by chemical equations.
1. Briefly write down the condition of the problem and make a chemical equation.
2. Above the formulas in the chemical equation, write the data of the problem, write the number of moles under the formulas (determined by the coefficient).
3. Find the amount of a substance, the mass or volume of which is given in the condition of the problem, using the formulas:
M/M; \u003d V / V m (for gases V m \u003d 22.4 l / mol).
Write the resulting number above the formula in the equation.
4. Find the amount of a substance whose mass or volume is unknown. To do this, reason according to the equation: compare the number of moles according to the condition with the number of moles according to the equation. Proportion if necessary.
5. Find the mass or volume using the formulas: m = M ; V = V m .
This algorithm is the basis that the student must master so that in the future he can solve problems using equations with various complications.
Tasks for excess and deficiency.
If in the condition of the problem the quantities, masses or volumes of two reacting substances are known at once, then this is a problem for excess and deficiency.
When solving it:
1. It is necessary to find the amounts of two reacting substances according to the formulas:
M/M; = V/V m .
2. The resulting numbers of moles are inscribed above the equation. Comparing them with the number of moles according to the equation, draw a conclusion about which substance is given in deficiency.
3. By deficiency, make further calculations.
Tasks for the share of the yield of the reaction product, practically obtained from the theoretically possible.
According to the reaction equations, theoretical calculations are carried out and theoretical data are found for the reaction product: theor. , m theor. or V theor. . When carrying out reactions in the laboratory or in industry, losses occur, so the practical data obtained are practical. ,
m practical or V practical. is always less than theoretically calculated data. The yield fraction is denoted by the letter (eta) and is calculated by the formulas:
(this) = pract. / theor. = m practical. / m theor. = V practical. / V theor.
It is expressed as a fraction of a unit or as a percentage. There are three types of tasks:
If the data for the starting substance and the share of the yield of the reaction product are known in the condition of the problem, then you need to find the practical. , m practical or V practical. reaction product.
Solution order:
1. Calculate according to the equation, based on the data for the original substance, find the theory. , m theor. or V theor. reaction product;
2. Find the mass or volume of the reaction product, practically obtained, according to the formulas:
m practical = m theor. ; V pract. = V theor. ; practical = theor. .
If in the condition of the problem the data for the starting substance and practice are known. , m practical or V practical. of the obtained product, while it is necessary to find the share of the yield of the reaction product.
Solution order:
1. Calculate according to the equation, based on the data for the starting substance, find
Theor. , m theor. or V theor. reaction product.
2. Find the share of the yield of the reaction product using the formulas:
Prakt. / theor. = m practical. / m theor. = V practical. /V theor.
If in the condition of the problem are known pract. , m practical or V practical. of the resulting reaction product and the share of its yield, in this case, you need to find data for the starting substance.
Solution order:
1. Find theor., m theor. or V theor. reaction product according to the formulas:
Theor. = practical / ; m theor. = m practical. / ; V theor. = V practical. / .
2. Calculate according to the equation, based on theor. , m theor. or V theor. reaction product and find data for the starting material.
Of course, we consider these three types of problems gradually, we work out the skills of solving each of them using the example of a number of problems.
Problems on mixtures and impurities.

A pure substance is that which is more in the mixture, the rest is impurities. Designations: mass of the mixture - m see, mass pure substance- m q.v., mass of impurities - m approx. , mass fraction of a pure substance - h.v.
The mass fraction of a pure substance is found by the formula: h.v. = m q.v. / m see, express it in fractions of a unit or as a percentage. We distinguish 2 types of tasks.
If in the condition of the problem the mass fraction of a pure substance or the mass fraction of impurities is given, then the mass of the mixture is given. The word "technical" also means the presence of a mixture.
Solution order:
1. Find the mass of a pure substance using the formula: m p.m. = q.v. m see.
If the mass fraction of impurities is given, then first you need to find mass fraction pure substance: h.v. = 1 - approx.
2. Based on the mass of a pure substance, make further calculations according to the equation.
If the condition of the problem gives the mass of the initial mixture and n, m or V of the reaction product, then you need to find the mass fraction of the pure substance in the initial mixture or the mass fraction of impurities in it.
Solution order:
1. Calculate according to the equation, based on the data for the reaction product, and find n hours. and m h.v.
2. Find the mass fraction of a pure substance in a mixture using the formula: q.v. = m q.v. / m see and mass fraction of impurities: approx. = 1 - h.c.
The law of volumetric ratios of gases.
The volumes of gases are related in the same way as their quantities of substances:
V 1 / V 2 = 1 / 2
This law is used in solving problems by equations in which the volume of a gas is given and it is necessary to find the volume of another gas.
The volume fraction of gas in the mixture.
Vg / Vcm, where (phi) is the volume fraction of gas.
Vg is the volume of gas, Vcm is the volume of the mixture of gases.
If the volume fraction of the gas and the volume of the mixture are given in the condition of the problem, then, first of all, you need to find the volume of the gas: Vg = Vcm.
The volume of the mixture of gases is found by the formula: Vcm \u003d Vg /.
The volume of air spent on burning a substance is found through the volume of oxygen found by the equation:
Vair \u003d V (O 2) / 0.21
Derivation of formulas of organic substances by general formulas.
Organic substances form homologous series that have common formulas. This allows:
1. Express the relative molecular weight in terms of the number n.
M r (C n H 2n + 2) = 12n + 1 (2n + 2) = 14n + 2.
2. Equate M r expressed in terms of n to the true M r and find n.
3. Compose reaction equations in general view and perform calculations on them.
Derivation of formulas of substances by combustion products.
1. Analyze the composition of the combustion products and draw a conclusion about the qualitative composition of the burnt substance: H 2 O -> H, CO 2 -> C, SO 2 -> S, P 2 O 5 -> P, Na 2 CO 3 -> Na, C.
The presence of oxygen in the substance requires verification. Designate the indices in the formula as x, y, z. For example, CxHyOz (?).
2. Find the amount of substances of combustion products using the formulas:
n = m / M and n = V / Vm.
3. Find the amounts of elements contained in the burnt substance. For example:
n (C) \u003d n (CO 2), n (H) \u003d 2 ћ n (H 2 O), n (Na) \u003d 2 ћ n (Na 2 CO 3), n (C) \u003d n (Na 2 CO 3) etc.
4. If a substance of unknown composition burned out, then it is imperative to check whether it contained oxygen. For example, СxНyОz (?), m (O) \u003d m in-va - (m (C) + m (H)).
b) if relative density is known: M 1 = D 2 M 2 , M = D H2 2, M = D O2 32,
M = D air. 29, M = D N2 28, etc.
1 way: find the simplest formula of a substance (see the previous algorithm) and the simplest molar mass. Then compare the true molar mass with the simplest and increase the indices in the formula by the required number of times.
2 way: find the indices using the formula n = (e) Mr / Ar (e).
If the mass fraction of one of the elements is unknown, then it must be found. To do this, subtract the mass fraction of another element from 100% or from unity.
Gradually, in the course of studying chemistry in the chemical dictionary, there is an accumulation of algorithms for solving problems of different types. And the student always knows where to find the right formula or the right information to solve the problem.
Many students like to keep such a notebook, they themselves supplement it with various reference materials.
As for extracurricular activities, the students and I also start a separate notebook for writing algorithms for solving problems that go beyond school curriculum. In the same notebook, for each type of task, we write down 1-2 examples, they solve the rest of the tasks in another notebook. And, if you think about it, among the thousands of different tasks encountered in the exam in chemistry in all universities, one can distinguish tasks of 25 - 30 different types. Of course, there are many variations among them.
In developing algorithms for solving problems in optional classes, A.A. Kushnarev. (Learning to solve problems in chemistry, - M., School - press, 1996).
The ability to solve problems in chemistry is the main criterion for the creative assimilation of the subject. It is through solving problems of various levels of complexity that a chemistry course can be effectively mastered.
If a student has a clear idea of all possible types of problems, has solved a large number of problems of each type, then he is able to cope with passing the exam in chemistry in the form of the Unified State Examination and entering universities.
Chemistry, like any science, requires precision. The data representation system in this field of knowledge has been developed for centuries, and the current standard is an optimized structure containing all necessary information for further theoretical work with each specific element.
When writing formulas and equations, it is extremely inconvenient to use integers, and today one or two letters are used for this purpose - the chemical symbols of elements.
Story
In the ancient world, as well as in the Middle Ages, scientists used symbolic images to denote various elements, but these signs were not standardized. It was not until the 13th century that attempts were made to systematize the symbols of substances and elements, and from the 15th century, newly discovered metals began to be designated by the first letters of their names. A similar naming strategy is used in chemistry to this day.
The current state of the naming system
To date, more than one hundred and twenty chemical elements are known, some of which are extremely problematic to find in nature. It is not surprising that even in the middle of the 19th century, science knew about the existence of only 63 of them, and there was neither a single naming system nor an integral system for presenting chemical data.
The last problem was solved in the second half of the same century by the Russian scientist D. I. Mendeleev, relying on the unsuccessful attempts of his predecessors. The naming process continues today - there are several elements with numbers from 119 and above, conventionally indicated in the table by the Latin abbreviation of their serial number. The pronunciation of the symbols of chemical elements of this category is carried out according to the Latin rules for reading numerals: 119 - ununenny (lit. "one hundred and nineteenth"), 120 - unbinilium ("one hundred and twentieth") and so on.
Most of the elements have their own names, derived from Latin, Greek, Arabic, German roots, in some cases reflecting the objective characteristics of substances, and in others acting as unmotivated symbols.
Etymology of some elements
As mentioned above, some names and symbols of chemical elements are based on objectively observable features.
The name of phosphorus, glowing in the dark, comes from the Greek phrase "bring light". When translated into Russian, quite a lot of "speaking" names are found: chlorine - "greenish", bromine - "bad smelling", rubidium - "dark red", indium - "indigo color". Since the chemical symbols of the elements are given in Latin letters, the direct connection of the name with the substance for a Russian speaker usually goes unnoticed.
There are also more subtle naming associations. So, the name of selenium comes from the Greek word meaning "Moon". This happened because in nature this element is a satellite of tellurium, whose name in the same Greek means "Earth".

Niobium is named similarly. According to Greek mythology, Niobe is the daughter of Tantalus. The chemical element tantalum was discovered earlier and is similar in its properties to niobium - thus, the logical connection "father-daughter" was projected onto the "relationship" of chemical elements.
Moreover, tantalum got its name in honor of the famous mythological character not by chance. The fact is that obtaining this element in its pure form was fraught with great difficulties, due to which scientists turned to the phraseological unit “Tantalum flour”.
Another curious historical fact lies in the fact that the name of platinum literally translates as "silver", i.e. something similar, but not as valuable as silver. The reason is that this metal melts much more difficult than silver, and therefore for a long time it was not used and was not of particular value.
General principle of naming elements
When looking at the periodic table, the first thing that catches your eye is the names and symbols of the chemical elements. It is always one or two Latin letters, the first of which is capital. The choice of letters is due to the Latin name of the element. Despite the fact that the roots of words come from ancient Greek, and from Latin, and from other languages, according to the naming standard, Latin endings are added to them.
It is interesting that most of the characters will be intuitively understandable to a native Russian speaker: a student easily remembers aluminum, zinc, calcium or magnesium from the first time. The situation is more complicated with those names that differ in the Russian and Latin versions. The student may not immediately remember that silicon is silicium, and mercury is hydrargyrum. Nevertheless, you will have to remember this - the graphic image of each element is focused on the Latin name of the substance, which will appear in chemical formulas and reactions as Si and Hg, respectively.

To remember such names, it is useful for students to perform exercises like: "Make a correspondence between the symbol of a chemical element and its name."
Other ways of naming
The names of some elements come from Arabic and were "stylized" in Latin. For example, sodium takes its name from a root stem meaning "bubbling substance". Arabic roots can also be traced to the names of potassium and zirconium.

It also influenced German. From it come the names of such elements as manganese, cobalt, nickel, zinc, tungsten. The logical connection is not always obvious: for example, nickel is an abbreviation for the word meaning "copper devil".
In rare cases, the names were translated into Russian in the form of tracing paper: hydrogenium (literally "giving birth to water") turned into hydrogen, and carboneum into carbon.
Names and toponyms
More than a dozen elements are named after various scientists, including Albert Einstein, Dmitri Mendeleev, Enrico Fermi, Ernest Rutherford, Niels Bohr, Marie Curie and others.
Some names come from other proper names: the names of cities, states, countries. For example: moscovium, dubnium, europium, tennessine. Not all toponyms will seem familiar to a native speaker of the Russian language: it is unlikely that a person without cultural training will recognize the self-name of Japan in the word nihonium - Nihon (literally: the Land of the Rising Sun), and in hafnia - the Latin version of Copenhagen. Finding out even the name of your native country in the word ruthenium is not an easy task. Nevertheless, Russia in Latin is called Ruthenia, and it is in her honor that the 44th chemical element is named.

The names of cosmic bodies also appear in the periodic table: the planets Uranus, Neptune, Pluto, Ceres. In addition to the names of the characters of ancient Greek mythology (Tantalum, Niobium), there are also Scandinavian ones: thorium, vanadium.
Periodic table
In the periodic table familiar to us today, bearing the name of Dmitry Ivanovich Mendeleev, the elements are presented in series and periods. In each cell, a chemical element is indicated by a chemical symbol, next to which other data are presented: its full name, serial number, distribution of electrons over layers, relative atomic mass. Each cell has its own color, which depends on whether the s-, p-, d- or f- element is highlighted.
Recording principles
When writing isotopes and isobars, a mass number is placed on the top left of the element symbol - the total number of protons and neutrons in the nucleus. In this case, the atomic number is placed at the bottom left, which is the number of protons.

The charge of the ion is written on the top right, and the number of atoms is indicated on the same side below. Symbols for chemical elements always begin with a capital letter.
National spelling options
The Asia-Pacific region has its own spellings of the symbols of chemical elements, based on local writing methods. The Chinese notation system uses radical signs followed by characters in their phonetic meaning. Symbols of metals are preceded by the sign "metal" or "gold", gases - by the radical "steam", non-metals - by the hieroglyph "stone".
In European countries, there are also situations when the signs of elements during recording differ from those recorded in international tables. For example, in France, nitrogen, tungsten and beryllium have their own names in the national language and are denoted by the corresponding symbols.
Finally
Studying at school or even higher educational institution, memorizing the contents of the entire periodic table is not required at all. In memory, you should keep the chemical symbols of the elements that are most often found in formulas and equations, and look at the little-used ones from time to time on the Internet or a textbook.

However, in order to avoid errors and confusion, it is necessary to know how the data is structured in the table, in which source to find the required data, and to clearly remember which element names differ in Russian and Latin versions. Otherwise, you can accidentally mistake Mg for manganese, and N for sodium.
To get practice at the initial stage, do the exercises. For example, specify the symbols for chemical elements for a randomly selected sequence of names from the periodic table. As you gain experience, everything will fall into place and the question of remembering this basic information will disappear by itself.
chemical signs
CHEMICAL SIGNS (chemical symbols) letter designations of chemical elements. They consist of the first or the first and one of the following letters of the Latin name of the element, for example, carbon - C (Carboneum), calcium - Ca (Calcium), cadmium - Cd (Cadmium). To designate nuclides, their chemical signs are assigned a mass number at the top left, and sometimes an atomic number at the bottom left, for example. Chemical signs are used to write chemical formulas.
Chemical signs
chemical symbols, abbreviated letter designations of chemical elements. Modern Z. x. (see table) consist of the first letter or the first and one of the following letters of the Latin name of the elements. In chemical formulas and chemical equations, each Z. x. expresses, in addition to the name of the element, the relative mass equal to its atomic mass. To designate isobars and isotopes to their Z. x. a mass number is assigned from above to the left (sometimes to the right); The atomic number is written at the bottom left. If they want to designate not a neutral atom, but an ion, then they put the charge of the ion at the top right. At the bottom right, the number of atoms of a given element in a molecule is indicated. Examples: ═≈ singly charged chlorine isotope ion (atomic number 17, mass number 35); ═≈ diatomic molecule of the same isotope. The isobars of argon and calcium are denoted by ═u, respectively. Given in the table Z. x. are international, but along with them, in some countries, signs derived from national names of elements are commonly used. For example, in France instead of Z. x. nitrogen N, beryllium Be and tungsten W are accepted Az (Azote), Gl (Glucinium) and Tu (Tungstène). In the United States, Cb (Columbium) is often used instead of Nb for niobium. The names and signs of elements with atomic numbers 102 and 103 (“nobelium” and “lawrencium”) are not generally accepted. History reference. Chemists ancient world and the Middle Ages, symbolic images, letter abbreviations, as well as combinations of both were used to designate substances, chemical operations and devices (see. rice.). The seven metals of antiquity were represented by the astronomical signs of the seven heavenly bodies : Sun (gold), Moon (silver), Jupiter (tin), Venus (copper), Saturn (lead), Mercury (mercury), Mars (iron). The metals discovered in the 15th-18th centuries - bismuth, zinc, cobalt - were denoted by the first letters of their names. The sign of wine spirit (lat. spiritus vini) is made up of the letters S and V. The signs of strong vodka (lat. aqua fortis, nitric acid) and golden vodka (lat. aqua regis, aqua regia, a mixture of hydrochloric and nitric acids) are made up of the sign of water Ñ and capital letters F, respectively R. The sign of glass (Latin vitrum) is formed from two letters V ≈ straight and inverted. Attempts to streamline the ancient Z. x. continued until the end of the 18th century. At the beginning of the 19th century The English chemist J. Dalton proposed that the atoms of chemical elements be designated by circles, inside which were placed dots, dashes, initial letters of the English names of metals, etc. Dalton received some distribution in Great Britain and in Western Europe, but were soon superseded by purely alphabetic Z. x., which the Swedish chemist I. Ya. Berzelius proposed in 1814. The principles he expressed for compiling Z. x. have retained their force to the present day; they are stated at the beginning of the article. In Russia, the first printed message about Z. x. Berzelius was made in 1824 by the Moscow doctor I. Ya. Zatsepin. Signs, names, atomic numbers and atomic masses of chemical elements Sign* Latin name Russian name Atomic number Atomic mass** Sign* Latin name Russian name Atomic number Atomic mass** Ac Actinium Actinium 89 [ 227] Mg Mgnesiom Magnesium 12 24.305 Ag Argentum Silver 47 107.8680 Mn Manganum Manganese 25 54.9380 Al Aluminum Aluminum 13 26.98154 Mo Molebdaenum Molybdenum 42 95.94 Am Americium Americium 95 N Nitrogenium Nitrogen 7 14.0067 Ar Argonum Argonium 18 39.9 .98977 As Arsenicum Arsenic 33 74.9216 Nb Niobium Niobium 41 92.9064 At Astatium Astatium 85 Nd Neodymium Neodymium 60 144.24 Au Aurum Gold 79 196.9665 Ne Neonum Neon 10 20.179 B Borum Boron 5 10.810 Ni Niccolum 58, 2 71 Ba Baryum Barium 56 137.34 (No) (Nobelium) 102 Be Beryllium Beryllium 4 9.01218 Np Neptunium Neptunium 93 237.0482 Bi Bismuthum Bismuth 83 208.9804 O Oxygenium Oxygen 8 15.9994 Bk Berkelium Berkelium 97 Os Osmium Osmium 76 190.2 Br Bromum Bromine 35 79.904 P Phosph orus Phosphorus 15 30.97376 C Carboneum Carbon 6 12.011 Pa Protactinium Protactinium 91 231.0359 Ca Calcium Calcium 20 40.08 Pb Plumbum Lead 82 207.2 Cd Cadmium Cadmium 48 112.40 Pd Palladium Palladium 46 Cerium 5 Cerium 140.12 Pm Promethium Promethium 61 Cf Californium California 98 Po Polonium Polonium 84 Cl Chlorum Chlorine 17 35.453 Pr Praseodymium Praseodymium 59 140.9077 Cm Curium Curium 96 Pt Platinum Platinum 78 195.09 Co Cobaltum Cobalt 24 Plutonium Cr Plutonium Chromium Chromium 24 51.996 Ra Radium Radium 88 226.0254 Cs Caesium Cesium 55 132.9054 Rb Rubidium Rubidium 37 85.4678 Cu Cuprum Copper 29 63.546 Re Rhenium Rhenium 75 186.2 Dy Dysprosium Dysprosium 66 162.50 Rh5 Rhodium102 9055 Er Erbium Erbium 68 167.26 Rn Radonum Radon 86 Es Einsteinium Einsteinium 99 Ru Ruthenium Ruthenium 44 101.07 Eu Europium Europium 63 151.96 S Sulfur Sulfur 16 32.06 F Fluorum Fluorine 9 18.99840 Sb Stibium Antimony 51 121, 75 Fe Ferrum Iron 26 55.847 Sc Scandium Scandium 21 44.9559 Fm Fermium Fermium 1 00 Se Selenium Selenium 34 78.96 Fr Francium Francium 87 Si Silicium Silicon 14 28.086 Ga Gallium Gallium 31 69.72 Sm Samarium Samarium 62 150.4 Gd Gadolinium Gadolinium 64 157.25 Sn Stannum Tin 50 118.69 Ge Germanium Germanium 32 72 .59 Sr Strontium Strontium 38 87.62 H Hydrogenium Hydrogen 1 1.0079 Ta Tantalum Tantalum 73 180.949 He Helium Helium 2 4.00260 Tb Terbium Terbium 65 158.9254 Hf Hafnium Hafnium 72 178.49 Tc Technetium Technetium 90 43 298 Hydrargyrum Mercury 80 200.59 Te Tellurium Tellurium 52 127.60 Ho holmium Holmium 67 164.9304 Th Thorium Thorium 90 232.0381 I Iodum Iodine 53 126.9045 Ti Titanium Titanium 22 47.90 In Indium Indium 49 114.82 Tl Thallium Thallium 81 204.37 Ir Iridium Iridium 77 192.22 Tm Thulium Thulium 69 168.9342 K Kalium Potassium 19 39.098 U Uranium Uranium 92 238.029 Kr Kryptonum Krypton 36 83.80 V Vanadium Vanadium 23 50.94 Kurchatov Kurtschatovim0 74 183.85 La Lanthanum Lanthanum 57 138.9055 Xe Xenonum Xenon 54 131.30 Li Lithium Lithium 3 6.941 Y Yttrium Yttrium 39 88.9059 (Lr) (Lawrencium) 103 Yb Ytterbium Ytterbium 70 173.04 Lu Lutetium Lutetium 71 174.97 Zn Zincum Zinc 30 65.38 Md Mendelevium Mendelevium 101 Zr Zirconium Zirconium 40 91.22 * In parentheses uncommon signs and names of elements with atomic numbers 102 and 103 are given. ** Atomic masses are given on the carbon scale (the atomic mass of the carbon isotope 12C is exactly 12) and correspond to the international table 197
- well.
- :
- scientific discipline studying substances, their composition, structure, properties and mutual transformations.
- Academic subject containing theoretical basis of this science.
- unfold A textbook that sets out the content of a given academic subject.
- The practical application of this science and its laws in production, industry, etc.
- The qualitative composition of smth.
- unfold Preparations, chemicals, solutions, etc. used in production and everyday life.
- unfold Food products containing almost no natural ingredients.
- trans. unfold Perm.
- :
The mass numbers of the longest-lived isotopes of radioactive elements are given in square brackets.
Lit .: Lomonosov M.V., Poln. coll. soch., vol. 2, M. ≈ L., 1951, p. 706≈709; Dzhua M., History of Chemistry, trans. from Italian., M., 1966; Crosland M. P., Historical studies in the language of chemistry, L., 196
Dictionary Ushakov
Chemistry
chi mia, chemistry, pl. No, female (Greek chemia). The science of composition, structure, changes and transformations, as well as the formation of new simple and complex substances. Chemistry, says Engels, can be called the science of the qualitative changes in bodies that occur under the influence of changes in the quantitative composition. Organic chemistry. Inorganic chemistry. Applied chemistry. Theoretical chemistry. Chemistry course.
| what. chemical properties of something scientific). Chemistry of oil.
encyclopedic Dictionary
Chemistry
(possibly from the Greek Chemia - Chemia, one of the oldest names for Egypt), a science that studies the transformations of substances, accompanied by a change in their composition and (or) structure. Chemical processes (obtaining metals from ores, dyeing fabrics, dressing leather, etc.) were used by mankind already at the dawn of its cultural life. In the 3-4 centuries. alchemy was born, the task of which was to turn base metals into noble ones. Since the Renaissance, chemical research has increasingly been used for practical purposes (metallurgy, glassmaking, ceramics, paints); there was also a special medical direction of alchemy - iatrochemistry. In the 2nd floor. 17th century R. Boyle gave the first scientific definition of the concept "chemical element". The period of transformation of chemistry into a true science ended in the 2nd half. 18th century, when the law of conservation of mass in chemical reactions was formulated (see also M. V. Lomonosov, A. Lavoisier). In the beginning. 19th century J. Dalton laid the foundations of chemical atomistics, A. Avogardo introduced the concept "molecule". These atomic and molecular concepts were established only in the 1960s. 19th century At the same time, A. M. Butlerov created the theory of the structure of chemical compounds, and D. I. Mendeleev discovered the periodic law (see. Periodic system elements of Mendeleev). From con. 19 - beg. 20th century the most important direction Chemistry was the study of the laws of chemical processes. In modern chemistry, its separate areas are inorganic chemistry, organic chemistry, physical chemistry, analytical chemistry, polymer chemistry have become largely independent sciences. At the junction of chemistry and other fields of knowledge, for example, biochemistry, agrochemistry, and geochemistry arose. The laws of chemistry are based on Technical science like chemical technology, metallurgy.
Ozhegov's dictionary
X And MIA, and, well.
1. The science of the composition, structure, properties of substances and their transformations. inorganic x. Organic x. Physical x. (based on general principles of physics).
2. what. The composition itself, the properties of substances and their transformations. H. carbohydrates. H. oil.
3. collected chemicals. Household x.
4. A way of influencing someone. with the help of chemicals (colloquial). Do chemistry (perm using such means). Take a chemistry course (i.e. a course of treatment with such agents, chemotherapy). Landings treated with chemistry (chemicals).
| adj. chemical, oh, oh.
Dictionary of Efremova
Chemistry
Encyclopedia of Brockhaus and Efron
Chemistry
The original meaning and origin of this word is unknown; it is possible that it is simply an old name for northern Egypt, and then Chemi science means Egyptian science; but since Chemi, in addition to Egypt, also denoted black, and μελάνοσις (blackening) was considered an operation inevitable in the transformations of metals, it may be that τέχνη τής χημείας - Olympiodorus, is the art of preparing this blackening substance (cf. H. Kopp , "Geschichte der Chemie", II, 1844, 4 - 6, and M. Berthelot, "Introduction a l" é tude de la chimie des anciens et du moyen вge ", 1889). "From most of the other sciences X. in its development is distinguished by the fact that its goal was understood differently at different times ... While in other areas of spiritual activity, whatever the attitude towards them in other periods, the goal was always clearly recognized, and it was steadily meant, in history of X. this is not observed at all. This science is changing not only the choice of auxiliary means and applications, but also the whole task, and the conditions for its existence (cf. Alchemy, Iatrochemists, Phlogiston) ... At the present time, - continues G. Kopp ("Geschichte der Chemie", I , 1843, 5), the task of X., taken by itself (an und f ü r sich), is the decomposition of compounds into constituent parts and the formation of constituent parts again compounds [This definition dates back to mid-seventeenth table., when Lemery, in his Cours de Chymie, says that "La Chymie est un art, qui enseigne a sé parer les differentes substances qui se rencontrent dans un mixte" (Corr. "Geschich." II, 8), and Stahl added to this "and the art of re-mixing the constituents" (Corr, l.c.). The concept of the constituent parts of mixtures has changed; the contemporary was already outlined by Boyle, but was generally accepted only after Lavoisier (see Lavoisier and Phlogiston).]. The task, therefore, is to know the composition of all bodies and precisely how they are formed and how they can be formed. natural history, the closest subject of which is "the study of homogeneous substances, from the addition of which all the bodies of the world are composed, their transformations and the phenomena accompanying such transformations. " According to Ostwald (W. Ostwald, "Grundlinien der anorg. Ch.", 1900, 1), "these transformations can be divided into two large, not quite strictly isolated groups. Sometimes transformations concern only one, or a few relations and properties of the body being studied; sometimes they are such that the body under study disappears as such, and new bodies with new properties appear in its place. Phenomena of the first kind are included in the field of physics, the second - in the field of X. ", and, as an example, Ostwald considers the ratio of sulfur to mechanical shocks (the relative position of the body changes, but does not change: color, weight, etc., so-called. its physical properties), to weak heating (they change - temperature, specific gravity and volume, vapor pressure, other (?) properties remain unchanged), to electrification and finds that phenomena of this kind should be considered physical. But "if you bring (l. s., 2) a piece of sulfur in contact with fire, it catches fire and burns with a blue flame. At the same time, the well-known smell of burning sulfur is felt, and after the combustion has been going on for some time, the sulfur, as such, disappears: it has burned out. In this process, not only the individual properties of sulfur change, but ... instead of it, something else was formed; we can judge this by the smell that appeared simultaneously with the beginning of the phenomenon, but was not noticeable before. In this case, sulfur participated in the chemical process ... The science of X. has the task of establishing the laws of all such transformations. "In other textbooks, physical transformations are defined as those in which the properties of matter remain unchanged, while restoring its original state; during the process moreover, it is impossible to divide a given homogeneous part of a transforming system into heterogeneous parts by any mechanical means, at least if we start from a physically homogeneous body; , the heating of ice, its melting, the transformation of the liquid water formed during boiling into steam are physical processes, because when the initial temperature (and pressure) is restored, the ice turns out to be in the same quantity with all the physical properties inherent in it under given conditions. properties; and although at the melting point of ice we can have the substance of water simultaneously in three states - solid (ice), liquid (water) and gaseous (steam) and we can mechanically separate them (ice can, for example, be filtered from liquid water), but neither ice, water, and steam cannot be further divided into physically heterogeneous substances by any mechanical means known to us. If, however, the ice is evaporated and the resulting vapor is heated to a temperature of 1500 ° - 2000 °, then by a mechanical process (using diffusion, see Dissociation) it is possible to isolate from the mass of superheated vapor a gas that differs from them in properties (a mixture of hydrogen and oxygen). By recooling, the water alone, unchanged, will turn into ice, and the gaseous body, collected separately and cooled rapidly, will retain its gaseous nature; it will therefore be an example of the chemical transformation of ice. Despite the fact that it is easy to find many more in textbooks similar examples , and despite the fact that the division of the transformations of matter into physical and chemical is consecrated by time, it is undoubtedly sharply one-sided, and therefore incorrect. Ostwald is wrong, if only because in his example he compares completely incomparable transformations. The changes in the properties of sulfur that occur in it when its "energy of position" is changed can be left aside; theoretically they are necessary, but in any case they are so insignificant that they are elusive not only with the help of our senses, but also with the help of the senses refined by the most sensitive modern instruments. When we weakly heat sulfur, we are dealing with the following phenomena. The system under study, which Ostwald calls sulfur, should be considered composed of two independent terms (see the Phase Rule): from sulfur and atmospheric oxygen [Nitrogen and all other gaseous constituents of it take too negligible a part in the transformation, except perhaps humidity - see Contact phenomena - and therefore their presence can be ignored]; it is under such temperature conditions (supercooled), when, due to passive resistances, the interaction between these bodies is almost impossible, or, if it does occur, then at such an insignificant, close to zero, speed that we are completely unable to catch it. We can, therefore, consider the entire system as being in a state of false equilibrium (faux equilibre) of Duhem, otherwise unstable (cf. A. Gorbov, "Law of Phases", in the "Physico-mathematical Yearbook", II), changing the equilibrium conditions to complete transformation; sulfur, considered separately, i.e. - neglecting its infinitely slow reaction with oxygen, we can consider a monovariant system of one term (solid sulfur + steam in the presence of two external equilibrium factors: temperature and pressure), and it is known that the laws to which such a system is subject (see the Phase Rule, l.c.) are no different from the laws to which any monovariant system with any number of independent terms is subject, the system of combining CaO + CO 2 (or dissociating CaCO 3), for example. ; in a mechanical sense, solid sulfur with its vapors form an indifferently stable system. But let us heat the sulfur + oxygen up to 500° approximately; immediately, their interaction begins along the contact surface, accompanied by the appearance of light and heat (the system was supercooled): sulfur, as they usually say, burns, but oxygen burns equally, meeting with sulfur vapor; for both terms, the measure of stability upon mutual contact was surpassed by heating, and the system became unstable, and it is obvious that it is illegal to bring together the indifferently stable state of sulfur with the unstable state of its own + oxygen; and while sulfur remained in an indifferently stable state, then, we repeat once again, the physical changes in its properties obeyed the same law as the "chemical" transformation in the CaO + CO 2 system. With a very slight change, what has been said is also applicable to the system of heated: ice, liquid water and its vapors. As long as ice and liquid water are heated alone, until then, for a given volume of the system, it is possible (at a whole range of temperatures and pressures) to coexist between two phases: ice + steam, ice + liquid water, liquid water + steam; all these systems are monovariant and, as such, do not differ in any way from dissociating chalk, from the formed (dissociating) iodine trichloride (see the Phase Rule, l.c.), i.e. from systems for which it is usually assumed that occurring in transformations of them are not physical, but chemical in nature. But we have superheated the water vapor by means of a special technique (diffusion) [In this way a new factor is introduced into the equilibrium conditions of the system, namely, capillary tension, and it is very possible that this changes the nature of the equilibrium (cf. the following note).] we managed to separate part of such a system, and we we assume that the remaining, unseparated mass of steam differs in physical properties from the separated part, that it only differs from ordinary steam in a different, higher energy content; but, obviously, this is only an assumption, although perhaps the simplest and most probable; as for the supercooled "explosive mixture", it cannot be compared with water, because such a comparison would be as unfortunate as comparing supercooled water with ice of the same temperature; one system (supercooled water) is unstable, with passive resistances (according to Gibbs), the other is indifferently stable, at least in the presence of two external equilibrium factors: temperature and pressure [We will arrange a Grove gas battery from hydrogen, oxygen and water, i.e. We will introduce several additional equilibrium factors into it, and it will become equilibrium, and its transformations will be reversible even at ordinary temperature.]. Summarizing the foregoing, we come to the conclusion that the usual definitions of X. are somewhat narrow, and the more general one is: X. is an exact science of natural history that studies the laws of changes in the state of matter [This does not prejudge the question of the unity or complexity of this matter.] ; it classifies them around "chemical" compounds, and these latter - around special, stable varieties of matter, called "elements" (for the meaning of the expressions "chemical compound" and "element" - see below the law of constancy of composition). It is possible, in this study, to call reversible changes in the state of matter physical and to distinguish them from those “chemical” transformations that, under our conditions, are irreversible and proceed one-sidedly, but we must remember that until recently and between these transformations, a part is recognized as physical, such, for example, , the transition of supercooled liquids to a solid state, the crystallization of supersaturated solutions [If such solutions are considered not from the point of view of the concentration of independent terms, but from the point of view of the influence of temperature on them, as an external equilibrium factor, then they should also be recognized as supercooled systems.], although they are nothing do not differ from "chemical" phenomena, which are: the explosion of liquid hydrogen peroxide, liquid ozone, an explosive mixture (hydrogen with oxygen, chlorine with hydrogen [Observations have shown that the mixture of oxygen with hydrogen is also affected by light, which accelerates the transformation.]), etc. e. From the above point of view, it is clear that the information usually reported in chemistry is one-sided and sketchy, and that numerous data should be attached to them, usually included in physics courses, crystallography courses, etc. etc., and which only recently entered the manuals of the so-called. physical chemistry. The planned evolution began relatively recently, and it is impossible to foresee the volume of X. even in the near future, but to a certain extent Mach is right when he says that "many relationships between physics and X. have been discovered in recent times. The old idea that X. can be considered as applied physics, in particular applied mechanics, received new encouragement in this ... In the absence of a prejudiced view, it seems more likely that X. of the future will embrace physics, and not vice versa "(Prinzipien der Wärmelehre", 1900, 5, 354); Undoubtedly, both sciences will gain in homogeneity if all those departments in which changes in the state of matter are studied depending on changes in its energy supply are transferred from physics to X.
Laws and hypotheses X. The basic laws of X. can be divided into general qualitative and general quantitative. quality laws.
I. Between them in the foreground should be placed Gibbs phase law; it has already been stated earlier (see the Rule of phases, l.c.) and here we can restrict ourselves to indicating that its most general expression is:
v = n + e - r,
where v- the number of independent variations of external and internal factors of system equilibrium or the number of its degrees of freedom; n- the number of its independent terms (internal equilibrium factors), or the number of those bodies whose concentration can be independently changed; e- the number of external factors of equilibrium (these are: temperature, pressure, capillary tension, electroexcitatory force, various stresses of gravity, etc.); r- the number of phases, i.e. physically different states of matter, separated (r - 1) by the number of interfaces. This expression follows from the articles of Gibbs himself, but was first written by Wald ("Zeitschrift f. Ph. Ch." 18, 1895, 346), and therefore, in words (cf. A. Gorbov, "Law of Phases", "Physic. Mat . Yearly", II) that each new body entering the system, and each new external factor of its equilibrium, increase by one the degree of freedom of the system (the number of possible phases, possible independent variations in temperature, pressure, etc.), and each new phase or newly formed interface lowers this degree of freedom by 1. The law of phases is an invaluable guiding thread in the study of the transformations of matter.
II. The second general qualitative law that determines the direction of transformation is Gibbs-Le Chatelier's law , which states that "any change in any factor of equilibrium entails a transformation in the system, which tends to cause in this factor a change opposite in sign to that which is communicated to it." This law is also stated earlier (see Reversibility of chemical reactions).
Quantitative, weight laws.
I. Law of conservation of mass of matter expressed by Lavoisier in a priori form: “We can recognize as an axiom,” he says, “that with all transformations, both artificial and natural, nothing is created anew: the same amount of matter exists before the experience and after it [Debus ("U é ber einige Fundamentalsatze der Chemie etc.", 1894, 6) considers Democritus of Abdera to be the founder of such a conviction, who taught that nothing can only come out of nothing and nothing that exists can turn into nothing; quoted by Aristotle in his Physics (I, 4)]. On this principle rests the possibility of all chemical experiments, and we are compelled by it to always expect a real identity, or equality, between the essences of the bodies studied and those that can be extracted from them by analysis" (Lavoisier, "Oeuvres etc." I , 101); there is no doubt, however, that this position was the result of Lavoisier's numerous experimental observations (see Phlogiston, Formulas and Chemical nomenclature). Since, for a given point on the globe, the masses of any bodies are strictly proportional to their weights, we can say that, according to Lavoisier's law: in any transformation, the weight of the transforming bodies is strictly equal to the weight of the bodies formed, and it is easy to see that this "chemical" law is a special case another, more general, to which all movements of matter are subject, and consisting in the fact that every time the mass of a given body changes (increases or decreases), then the mass of one or more surrounding bodies undergoes a simultaneous change, equal in magnitude, but of the opposite sign (decreases or increases)[Gaultier and Charpy "Le ç ons de Chimie", 1900, 14] [The law of conservation of mass of matter is quite parallel to the law of conservation of energy in physics (cf. B. Stevarta. P. G. Tait, "Unseen Universe", 1890).]. When Stas synthesized silver iodide and bromide from weighed amounts of silver, iodine and bromine, the weight of halogen compounds turned out to be, however, somewhat less than silver and iodine, silver and bromine, weighed separately; in addition, L. Meyer ("Moderne Theorien d. Ch.", 1884, 135) indicated the possibility that the particles of our ponderable matter are connected with a greater or lesser amount of not quite weightless light ether, the amount of which, perhaps, changes with chemical transformations; in view of this, first Landolt, and after him Heidweiler, subjected Lavoisier's law to a thorough experimental test; both studied the weight changes of various systems enclosed in sealed glass vessels. Landolt found that the weight of the system: an aqueous solution of silver sulphate + a solution of ferrous sulfate acidified with sulfuric acid decreases during the reaction:
Ag 2 SO 4 + 2FeSO 4 + H 2 SO 4 = 2Ag + Fe 2 (SO 4) 3 + H 2 O
per 0.130 mg - 0.167 mg; this decrease exceeds the weighing error by 6 - 12 times, but it is disproportionate to the reacting masses, since it was = 0.130 mg at 171.3 g and 0.167 mg at 114.2 g of the reacting system; in the reaction of iodic acid. with hydrogen iodide in the presence of sulfuric acid:
HJO 3 + 5H 2 SO 4 + 5KJ \u003d 3J 2 + 5KHSO 4 + 3H 2 O
a decrease in weight was also observed, but the difference (0.011 mg - 0.047 mg) lay within the experimental error; during the reaction of iodine with an aqueous solution of sodium sulfide salt (the interaction can go in two directions:
J 2 + 2Na 2 SO 3 \u003d 2NaJ + Na 2 S 2 O 6
J 2 + Na 2 SO 3 + Η 2 Ο \u003d 2HJ + Na 2 SO 4,
chloral hydrate with caustic potash
[CCl 3 .CH (OH) 2 + KOH \u003d CCl 3 H + SNKO 2 + H 2 O]
and when chloral hydrate was dissolved in water, no change in weight was observed that did not fall within the experimental error. Heidweiler studied the following transformations: the displacement of copper by iron in acidic, basic (?) and neutral solutions copper sulphate, dissolution of copper sulphate in water, dissolution of its acidified - in water and medium - in sulfuric acid, precipitation of copper oxide hydrate with caustic potash from a solution of copper sulphate, interaction of ammonia with acetic acid and precipitation of barium chloride with sulfuric acid. With a total number of responding bodies of about 200 g (160 - 280) and with a weighing error not exceeding 0.04 mg, in two cases he observed a gain in weight of 0.014 and 0.019, and in the remaining 21 decreases in weight ; in 13 experiments it was greater than the possible error and once reached 0.217 mg; with no doubt, a decrease was established during the precipitation of copper in an acidic and alkaline solution (but not in a neutral one), during the dissolution of acidified copper sulphate in water and during the precipitation of copper oxide hydrate [In 2 experiments, however, a decrease was too insignificant, namely 0.037 and 0.032 mg]. Heidweiler could not figure out the reason for the change in weight, and besides, the weight loss was not proportional to the mass of the reacting bodies. Thus, it turns out that, during certain transformations, the mass of the transformed matter seems to decrease, and this decrease lies outside the limits of weighing errors; it cannot be explained (Landolt) by the different tension of universal gravitation with respect to equal masses of different bodies, since the experiments of Bessel with pendulums made of various metals and minerals and Eötvös (E ötvö s) with torsion balances showed that such a difference cannot be caught; on the other hand, the retreats seem to be out of proportion to the reacting masses, and this makes some chance error probable; as long as one can, it seems, continue to consider Lavoisier's law, within the accuracy of modern methods of observation, perfectly accurate. In any case, errors like the above cannot be taken into account in ordinary experiments [In order for a system of basic copper sulphate with iron to lose 1 pood in weight after the reaction, it is necessary, judging by Heidweiler's data, to take in the most favorable case a little more than 1,000,000 poods. . mixtures. Most recently, Heidweiler reported (Physikalische Zeitschiift, 1902) that the weight of radium in a sealed tube decreases by 0.02 mg per day, and it is remarkable that the decrease in potential energy due to this (= K×[(M Δt)/r 2 ]×r, where K fast., M earth mass, r- its radius, Δt change in the mass of a body attracted by the Earth) = 0.02.600000000 mg cm = approx. 12.10 ergs, i.e., just the energy emitted, according to Becquerel, by radium per day. Heidweiler's report is preliminary.].
II. The law of constancy of the composition of chemical compounds which can be formulated as follows: the masses of bodies that, by their combination, form a new body that has a given sum of physical and chemical properties, are in constant relation both to each other and to the mass of the formed body, is usually considered the most characteristic of chemistry; it is sometimes even defined as a science that studies the composition and transformations of only homogeneous bodies, that is, those that are characterized by a constant composition, which represent real chemical individuals, and which are given the name of certain chemical compounds, in contrast to mechanical mixtures and indefinite chemical ones ( ?) compounds (see Tikhvinsky, "Method and system of modern chemistry", St. Petersburg, 1900, 3 and 6). On the other hand, one can find a comment about this law (Gautier et Charpy, l.c., p. 14) that "it represents nothing but a tautology. Indeed, there is no other definition of a "certain" connection, besides that which is derived from this so-called law Physical properties are not enough to characterize a compound, so we observe quite definite properties for a mixture of water and alcohol, taken in a certain ratio (by weight), although no one has ever therefore, there is no real law here, but a statement of a fact, however, a very remarkable one. Namely, many elements can form complex bodies only by combining in certain proportions, which remain unchanged, whatever the way of obtaining a complex body; if one of the elements is in excess, then it will remain as such after the act of union. Wald says even sharper (Zeitsch. f. ph. Ch., 1897, 22, 256): “The law of constancy of composition must be regarded as an empirical law. But even this is not entirely correct. if some substance, which was considered a chemical compound - and this is not so rare - turns out to change its composition with changing conditions? Will he doubt the correctness of the law? Obviously not; he will only strike the substance off the list of chemical compounds ... The point is that there are no other signs to recognize a substance as a chemical compound ... So, it has been learned by experience that some complex bodies have a constant composition. The recognition that all such substances, and only they alone, should be considered chemical compounds, is arbitrary. Therefore , chemical compounds have a constant composition by virtue of the definition, and by definition, those bodies that do not satisfy this condition are not recognized as chemical compounds. In view of the foregoing, it seems interesting to find out in what relation the law of constancy of composition is to Lavoisier's law, the history of its occurrence, and what we should currently consider a mechanical mixture, indefinite and definite chemical compounds. Lavoisier's law requires that the mass of reacting bodies be equal to the mass of the new body formed from them, but does not prejudge the number of reacting bodies at all; any number of them, as long as they are greater than zero, satisfy him; Lavoisier's law does not prejudge the question of whether bodies cannot react in innumerable ways; the law of constancy of composition says that a reaction is possible only at a certain specific ratio of reacting masses, but also does not give indications as to the number of possible compounds. It is remarkable that chemists have long been instinctively convinced of the constancy of the composition of the bodies they study; it suffices to point out that the composition of salts was determined by: Bergman (between 1775-1784); Wenzel (1777), Kirwan and Richter (1790-1800); that Lavoisier, having determined the composition of carbon dioxide and water, began to study the composition organic compounds, which he burned for this, collected the resulting water and carbon dioxide, and calculated the content of carbon and hydrogen in the burned substance, etc .; and this, obviously, would be impossible if he allowed that the composition of water and carbon dioxide could change. Thus, the belief in the constancy of the composition of complex bodies existed for a long time, or rather, no one suspected the possibility of anything else, but the "law" remained unspoken. His decisive opponent was Berthollet ("Recherches sur les lois de l" afnnt é", 1801 and 1802 and "Essai de statique chimique", 1803). He was convinced that bodies can sometimes be connected in all sorts of ways, sometimes in known limits; he saw the reason for this limitation in the fact that the force with which the constituent parts are held in a complex body must decrease with an increase in the mass of one of the reacting bodies (as it approaches a state of saturation and a relative decrease in the mass of the other), and secondly , in the influence of temperature on cohesion and on the natural elasticity of reacting bodies Thanks to the high authority of Berthollet, thanks to the wit with which these views were stated, they gained many supporters, especially since the analytical data then available were in many ways a direct confirmation of the correctness of such views. Proust (Proust, see the corresponding article) was an opponent of Berthollet's ideas [Proust is credited in this article with the idea of the origin of chemical elements from about bottom primary matter, namely hydrogen, but this idea was expressed by the English physician Prout (Prout) (see) and Weight of atoms (see).]; in a number of works (1801-1808) he showed that the formation of oxides, sulfur compounds and salts, in general, is associated with certain and invariable relations between the masses of the elements found in them, but what is visible only if we distinguish mechanical and other physically and chemical heterogeneous mixtures from chemical compounds. The law of the constancy of the composition of the latter, namely oxides, was expressed by Proulx in 1801 in the following words (Corr, "Geschichte d. Ch.", II, 368): "Always unchanged proportions, these constant attributes, characterize real compounds, both artificial and natural, in a word, this pondus naturae, which Stahl so clearly sees; all this, I say, is no more in the power of the chemist than the electoral law to which all compounds are subject. "Definite" compounds can, according to Proulx, mix with each other in indefinite ones. relations, but the product of such mixing is not a chemical compound, but a solution. Berthollet considered (in his "Statique chimique") that Proulx's views had little foundation, and a dispute broke out between them, which ended in 1808, when the majority of his contemporaries leaned on Proulx's side, after which an intensive study of certain chemical compounds began. At present, it is certain that the issue should be revisited. In order to give an idea of the modern point of view, let us dwell on the simplest case of the interaction of any two bodies that do not form between themselves what is called a definite combination, but are capable, under certain conditions, of forming liquid and homogeneous systems in all directions. As is well known (cf. Phase Rule, Alloys, Fractionated Evaporation), the addition of a body AT to the body BUT BUT, and body addition BUT to the body AT causes a decrease in temp. body melting AT, and therefore, when applying all kinds of mixtures formed by these two bodies, on a diagram of temperatures and concentrations, we get two curves intersecting at the eutectic point, emanating from the melting temperature BUT and AT(see pic.):
A detailed study of the diagram shows the following. Over curves CE and ED we have the area of liquid systems, usually called a solution AT in BUT (BUT melts much lower B) but which, obviously, are also solutions BUT in AT. Above the horizontal dotted line starting from the dot D, both bodies mix as liquids in every way (from 100% BUT up to 100% AT); between this line and the horizontal dotted line starting at the point WITH, body BUT, liquid under these conditions, can be added to the solution in an indefinite amount without violating its homogeneity, and the addition of a body AT limited by its solubility curve DE; the solution due to this is, as it were, one-sided. Below the horizontal dotted line starting at the point WITH, both solids but have a limited ability to melt each other; solution is symmetrical. Below the dotted line ab both bodies can be taken in any relationship, but they have no influence on each other; they are absolutely indifferent even with a further decrease in temperature, and we are not able to bring them into interaction under these conditions (external equilibrium factors of the system are assumed to be temperature and vapor pressure A + B). In a triangle CaE precipitates in the solid state excess solid A, in contact and balance with the body saturated with it A, solution; in a triangle DbE precipitates in a solid state b, also in contact and equilibrium with a solution saturated with it. What lies in the rectangle AaBb we usually call mechanical mix, although in fact there is no mixing of the bodies taken here [By denying the mixing of bodies, we mean their indifferent relation to each other and their complete spatial isolation. There is no doubt that some eutectic metal conglomerate (see Alloys) gives the impression of a homogeneous body to the naked eye with a microscope.]; they are as mixed as if they were in separate devices; therefore, it is more correct to call such a "mechanical" mixture together with B. Rooseboom (see Stereoisomerism) a conglomerate; the constituent parts of a conglomerate can be separated from each other by various methods and, among other things, with the help of heavy liquids (the method of Church and Thule in mineralogy). The composition of such a conglomerate can vary from almost 100% BUT up to 100% b, but it is obvious that for any given mixture it will, under a whole series of changes in temperature, remain constant; and whether we consider it a definite compound or not will depend on the greater or lesser ease with which we can prove its physical inhomogeneity at various points in the system and on the greater or lesser availability of the eutectic point for us E, above which the heterogeneity of the conglomerate will have a clearer effect (in the solid state they will be the body BUT or body AT), unless its concentration accidentally corresponds to the eutectic point, when and above its substance will be treated as completely homogeneous, for which the eutectic temperature will be the melting point [That such a conglomerate melts at the eutectic temperature into a homogeneous liquid is proved by the experiments of Gallock (1888), who found, that a conglomerate of sawdust of cadmium (1 hour), tin (1 hour), lead (2 hours) and bismuth (4 hours), corresponding in composition to Wood's alloy, melts in a water bath (with a sufficiently long heating), i.e. e. below 100°, while the individual metals melt: Cd at 320°, Sn at 32°, Pb at 320° and Bi at 269.2°; he also found that it was enough to press potassium (pl. at 62.5 °) and sodium (pl. at 97.6 °) to each other with fresh surfaces to get them liquid at ordinary. pace. and a mercury-like alloy (solution).]. Then bodies BUT and AT, precipitated in solid form from solution will also have an unchanged composition, since it is assumed that they can melt without decomposition (changes in composition) and, in addition, it is assumed that we have such a case of their interaction when only their concentration changes when they go into solution per unit volume, but not the composition [Actually speaking, such an ideal case does not actually take place: and the crystals of the body BUT, and body crystals AT fall out, moistened with a saturated solution, the composition of which changes with temperature and may even differ due to capillarity, in composition from the rest of the liquid mass. Such a solution, however, is relatively easy to remove, and this is the reason for the representation presented in the text. That the crystals of ice precipitated from "weak" aqueous solutions do not represent solid solutions is clear from Regnault's data on the vapor pressure of such solutions, and from some of Rueddorf's observations on weak aqueous solutions of pleochroic salts.]. Finally, the solution will have a variable concentration as long as its composition corresponds to the area lying above the lines CE and ed, and as long as one of the external equilibrium factors, temperature (at constant pressure) or pressure (at constant temperature), the system will change; but how soon do we have a solution corresponding to one of the boundary curves G.E. or ed, i.e. one of two possible monovariant systems, and the value of the temperature or pressure of the system is given in advance, or as soon for the solutions lying above CE and ED and representing divariant systems, the values of temperature and pressure are fixed, so the compositions of such solutions turn out to be completely fixed, definite, and it has long been known that the composition of saturated solutions is determined by the temperature and the nature and state of the solid body in contact with them, and that in order to in order to have an unsaturated solution of some bodies having at a given temperature a certain vapor pressure, a desired and possible specific gravity, a desired coefficient of refraction, etc., that for all this the reacting bodies must be taken in a strictly defined "constant weight ratio." Thus, we come to the conclusion that all invariant (nonvariant) systems have a certain composition [The reasoning applied in the text to a two-body system can be easily extended to a system of any complexity. The conglomerate lying below the eutectic temperature will not always consist of pure bodies. BUT and AT; The last case occurs when BUT and AT give connections. But it is not difficult to understand such cases, being guided by the foregoing and knowing the corresponding diagram; see, for example, the Fe 2 Cl 4 solubility diagram given by V. Rooseboom in Art. Fractionated evaporation.]; its constancy does not, therefore, represent the privilege of "certain, chemical" compounds, and therefore it is urgently necessary to find for "certain, chemical" compounds, the description of which so far constitutes almost the entire content of X., some sign other than the constancy of composition, which would allow characterize them. This sign was given by Wald, who defined a permanent chemical compound, as a phase of unchanged composition in a monovariant system. In the case discussed above, these phases are solids BUT and AT in contact with its saturated solutions: with an increase in the temperature of the latter, with a change in their pressure, the composition of the solution is constantly changing, and the solid phase, although it is constantly changing in quantity [The mass of the entire system is assumed to be constant.], but retains its unchanged composition, its individuality. There is no doubt that the sign indicated by Wald has long been known to chemists, and they constantly used it when discovering "permanent, chemical" compounds, but before Wald it was not clearly formulated by anyone, and the definition of "chemical" compounds in textbooks was therefore incomplete. In experiment, however, in order to establish the "homogeneity" of a substance, it has always been necessary to crystallize it from different "solvents" and at different temperatures, that is, to force it to play the role of a body AT our example; had to determine the weight of its vapor and compare the composition of the vapor with the composition of the liquid (solid) body, etc. What explains, or, more correctly, what does the circumstance that the bodies BUT and AT retain their composition unchanged over a range of temperature and pressure changes? The point is that if the bodies BUT and AT exothermic, they retain their composition as long as we study them at temperatures below those temperatures at which dissociation reactions can begin in them BUT on the a 1 and a 2 , V on the b 1 and b2; if BUT and AT under the conditions of the experiment, the compounds are endothermic, then they retain their individuality as long as we bring them into mutual contact above a certain limiting temperature, below which they can hardly exist, ready to disintegrate into their component parts [Under such conditions, there are usually all "endothermic" compounds, some of which are listed above. Recall that hydrogen peroxide, an “endothermic compound”, is formed in a flame of detonating gas, that Si 2 Cl 6 (Troost and Hautefeuille) is formed from SiCl 4 and Si above 1300 °:
begins to decompose below this temperature and is completely dissociated already at 800°. But if a gas heated to 1300° is suddenly cooled, then a liquid is obtained, kip. at 140° and beginning to decompose only at about 350°C; below it is preserved, thanks to passive resistances. Wed Phosphorus - on Tammann's researches on the conditions of transformations of supercooled (endothermic) systems.] Then they retain their individuality as long as we bring them into interaction at pressures greater than the dissociation pressures inherent in their decomposition reactions; or, finally, with endothermic systems, when we study them at such a degree of supercooling, when the transformation going on in them (if only it takes place) is practically imperceptible for us. Consequently, the constancy of the composition is established by the chosen conditions of the experiment. But why are compounds formed not in all possible proportions, but for the most part (cf. Hydrocarbons) in a very limited number of them? Wald responds to this by pointing out the limited mutual solubility of solids [In order to understand this for yourself, it is enough to study the solubility curves of calcium chloride hydrates (see the Rule of phases l. c.) or ferric chloride (see Fractionated evaporation l. c.) , where it is seen that the solubility of water in the taken halide salts in the solid state just corresponds to a very limited number of proportions.] and derives (l.c.) from this position even the law of multiple ratios (see below), but it is undoubted that, in addition to In addition, a limited number of compounds is also due to the so-called chemical nature of bodies, which makes, for example, that for hydrogen with oxygen, only water is the only stable (exothermic) compound under our conditions, and the remaining systems (H 2 O 2 , H 2 O 4 ?), containing more oxygen at our temperatures and pressures, are not very stable (supercooled) and can hardly be stored on a short time. Then, as can be seen from the examples just given, this limitation is apparent, due to randomly limited (“ordinary”) conditions under which we study the interactions of various bodies. But if cases of limited solubility are observed, then the opposite phenomenon should also be expected, i.e., cases of complete mixing of bodies in the solid state in all possible respects should be expected, otherwise, the formation of such systems, which, having the usual features of "chemical" compounds, will differ from them by the complete uncertainty of the composition. Some of the phenomena related to this are usually described as isomorphic mixtures (see the corresponding article), while some are described in general under the name of solid solutions (van "t Hoff, Mallard, Klein, Runne, Buxhoevden u. Tammann). Considering the interaction of bodies above BUT and AT from the point of view of the law of phases, we did not decide whether these bodies are elements, or whether they are "chemically" complex. The fact is that the law does not make any difference between the elements and their compounds, and it is equally applicable both to the phenomena of dissolution of calcium chloride hydrates in water (see the Phase Rule), and to the interaction of two elements, chlorine and iodine (l. c .). The only hitherto known difference between elements and compound bodies is that they were not tangibly decomposed into any forms of matter different from them, and therefore we still adhere to Lavoisier's definition (see Chemical nomenclature); the only difference is that in view of the law of Dulong and Petit (see Heat) and the periodic law of D. I. Mendeleev (see Periodic law of chemical elements), we can assert with a high degree of probability that all modern elements, if they are complex, then the complexity of them is of the same order ["We daily transform matter in every possible way. But at the same time, we have precisely determined the boundaries where such transformations stop: they have never crossed so far beyond ... chemical elements. This boundary is not indicated to us by any philosophical theory, this is a factual obstacle which we, with our methods of conducting experiments, were not able to overcome ... Does this mean, however, that mentally we see here the final limit. No, without a doubt; in fact, chemists have always looked to this border as an indisputable fact, but always with the hope of overstepping it. M. Berthelot, "Les origines de l" Alchimie "(1885).] Recently, many have already expressed the conviction that a simplification of our elements has been achieved; for example, J. J. Thomson believes that this assumption can only be the phenomena observed during the passage of cathode rays in rarefied gases are explained: “Since cathode rays carry negative charges; deflected by electrostatic forces as if they were negatively charged; are subject to the action of a magnetic force in exactly the same way as if this force acted on a negatively charged body moving along the path of these rays, then I see no way to avoid the conclusion that they represent negative electric charges carried by particles of matter. The question is, what are these particles? Do they represent atoms, molecules, or matter in a state of great separation? In order to shed some light on this circumstance, I made a series of measurements of the ratio of the mass of these particles to the charge they carry"; as a result, it turned out that m/e(m- weight, e- charge) does not depend on the nature of the gas and is very small (= 10 -7) compared to the smallest such value known so far, namely - 10 -4, which corresponded to the hydrogen ion during the electrolysis of aqueous solutions of acids, why Thomson concluded that in cathodic conditions "we are dealing with a new state of matter, such a state when its division is advanced much further than in the gaseous state; such a state when different types of matter, i.e., originating from hydrogen, oxygen, etc., become identical", etc. Despite numerous works in this area, the problem has moved relatively little forward due to experimental difficulties; therefore, it is only appropriate to outline it here and, by the way, cite Ostwald’s review, according to which “the basic law of electrolysis, Faraday’s law, turned out to be completely inapplicable to matter or bodies carrying current in gases. This contradiction is expressed in such a form that, supposedly, research over the conductivity of gases, they proved the existence of material particles several hundred times smaller than a hydrogen molecule (200 times); but the hypothetical nature of such a conclusion is obvious, and the name of ions for these phenomena, following completely different laws, is inappropriate "(1901). We have to wait for further experimental explanation of the subject.
III. Law of Equivalents (cf. Unitary system). Bergman had already noted that when solutions of two neutral salts are mixed, the neutrality of the solution is not disturbed, but he did not pay sufficient attention to this circumstance. The first one engaged in a thorough study of the phenomenon of Wenzel (1740-43), who laid the foundation for stoichiometry with his work "Vorlesungen über die chemische Verwandtschaft der Kö rper" (1777) (see). Confirming the correctness of Bergmann's observations, Wenzel gave an explanation of them, which consisted in the fact that different amounts of different alkalis and earths, neutralizing the same amount of any acid, should neutralize equal amounts of any other acids; in other words, that the ratio between the masses of two earths that neutralize a given amount of some acid remains constant even when they neutralize all other acids, and this made it possible to check the analyzes and even calculate the amount of some base necessary to form an average salt with a given acid, if the quantity of only one base required for this purpose was known; Wenzel himself, however, did not attach particular importance to this circumstance, and his work was not appreciated by contemporaries, although it was very accurate for that time. Was not happier and the closest follower of Wenzel - Richter. Richter began (1789-1802) by arranging in series the relative weights in which acids combine with bases to form neutral salts. The number of bases required to neutralize 1000 hours of sulfuric acid, he called the neutral series (Neutralit ä tsreihe) of bases; in the same way, he determined the neutral series of various acids necessary for the neutralization of given quantities of various bases. Despite the relatively low accuracy of his figures, Richter noticed that the numbers of neutral series of bases are proportional to each other and that the same is true for the neutral series of acids. In connection with these works, there is another "discovery" of Richter, namely, he owns extensive observations on the quantities in which metals displace (see. Displacement) each other from neutral salts, that is, the determination of those quantities in which they combine with a constant amount of oxygen, and in the case when metals are displaced from salts of one acid, and those quantities in which they, in the form of oxides, are combined with a constant amount of acid anhydride [To make this clear, it is enough to present copper sulphate as a compound copper oxide with sulfuric anhydride and write the equation for the displacement of copper by iron:
CuO.SO 3 + Fe = FeO.SO 3 + Cu;
it shows: from 16 wt. units oxygen are combined 63 wt. units copper and 56 wt. units iron (Cu = 63 and Fe = 56 in round numbers), and that (63 + 16) wt. units copper oxide and (56 + 16) wt. units iron oxides are combined with 80 wt. units sulfuric anhydride (S = 32 in round numbers)]. Previously, Bergman studied the mutual displacement of metals and published his observations in the article: "De div e rsa phlogisti quantitate in metallis". He found that in order to displace silver from its nitric acid salt, quite definite and constant quantities of other metals are required; then he studied the mutual displacement of metals from other salts; large differences were observed in the amounts of precipitating metals, but subject to constant laws. As a supporter of the phlogiston theory, Bergman looked at his figures in the following way: each metal, when dissolved, turns into "lime", i.e., loses the phlogiston contained in it (see); and since, when precipitated by another metal, it precipitates in a metallic state, it is undoubted that it is reduced, recombines with the amount of phlogiston necessary for it, at the expense of the metal that precipitates it, and Bergman, on the basis of his experiments, concluded that different metals 1) are connected with different amounts of phlogiston and 2) that the figures he obtained give those quantities of metals that contain equal amounts of phlogiston. Dec 20 1783 Lavoisier presented to the Academy a memoir Sur la précipitation des substances mé talliques les unes par les autres (Oeuvres etc., II, 528), where, pointing to Bergmann's results, he says that, "in his opinion, the absence or presence of phlogiston in metals is nothing but an assumption.In reality, however, and can be recognized with weights and measures in hand, that with any calcination of a metal, whether it occurs dry or wet, with the help of air, water or acids, there is observed an increase in the weight of a metal caused by the addition to it of ... oxygen (princip e oxygè ne) ... therefore, if 31 lb. of copper is sufficient to precipitate 100 lb. of silver in the metallic state [The real figure is 29.46 weight units copper per 100 weight units of silver; Bergman's experiments in this case were erroneous by about 4%.], which means that this amount of copper is able to combine completely with all the oxygen contained in 100 fn. silver ... in the state of lime "; Further, Lavoisier does not take into account the correct remark just made and, basing his calculations on the incorrect data of Bergman, comes to completely incorrect conclusions. A few years later, Richter's work appears with more accurate data and with an explanation devoid of the contradictions of Lavoisier's memoir. Richter establishes, incidentally, that mercury and iron form several definite compounds with oxygen, but he sets out the results of his work in a highly convoluted language, besides, they contain numerous calculations relating to a number of imaginary laws, which Richter thought that he opened. Almost all of these works go unnoticed, and the equality of the amount of oxygen is then again discovered by Gay-Lussac (in 1808), and the existence of various constant compositions of oxides of iron and mercury - by Proulx during his dispute (see corresponding article) with Berthollet. In 1782, Fischer drew attention to the work of Richter and found that all his tables of neutral series can be reduced to one, consisting of two rows: one contains the numbers of bases expressed in numbers, and the other contains the amounts of acids necessary for the formation of neutral salts with the indicated the number of bases. "These numbers expressed, consequently, the neutrality relations between bases and acids, and the table that concluded them summarized in a clear and convenient form the composition of a large number of neutral salts." Thanks to Fischer, the results of Richter's work became well known, but their influence was still very small, and what he found was subsequently rediscovered. Meanwhile, Wenzel and Richter discovered the fact that if two bodies are connected to a third in some respect A:B then they can also replace each other in the same ratio in a whole series of complex bodies, and in a particular case they can, consequently, in the same ratio or in a multiple of it (see below) be combined with each other. These characteristic numbers were named by Wollaston - equivalents; in modern textbook equivalents are defined as (proportional) numbers showing in what weight quantities the elements are combined with one weight. units hydrogen or replace it.
IV. Law of multiple ratios owned by Dalton; the history of its origin cannot now be reconstructed with accuracy; it is usually formulated like this: if two bodies A and B are connected in several ratios, then the masses of body B per the same mass of body A are in simple multiple ratios between themselves and at the same time in a simple and multiple ratio with the equivalent of body B; a more general formulation is due to Duhem (Le mixte et la combinaison chimique, 1902, 73): "Let C 1 , C 2 , C 3 ... there will be various elements; for each of them we can choose a characteristic number for it, called a proportional number ("atomic" weight) and obtain, next, a table of proportional numbers ("atomic" weights): p 1 , p 2 , p 3 ... If the bodies C 1 , C 2 , C 3 ... are interconnected, then the masses of the connecting bodies are in the relationship: λр l , μр m , νр n ... where λ, μ, ν are integers... Dalton and his contemporaries would not be satisfied with the expression "whole numbers", but would say "whole prime numbers"; but this limitation, correct at the beginning of chemistry, becomes less and less true as it develops; in particular, the progress of organic chemistry has forced in many cases to attribute to integers λ, μ, ν... big values; the character of simplicity, which at first was attributed to them, has thereby disappeared; how, for example, to find it in the formula of paraffin, where the masses of combined carbon and hydrogen are related as λ once taken proportional ("atomic") weight of carbon and μ once taken proportional weight of hydrogen, and where λ and μ have meanings: λ = 27, μ \u003d 56?" Indeed, the usual formulation of the law is not applicable not only to paraffins (see), where the ratio between the indicators in the formulas for the "proportional weights" of hydrogen and carbon is transmitted by a fraction 2+2/n, but in general to everyone unlimited series hydrocarbons, starting with the acetylene series, since it is sequentially equal to: 2 - 2/n, 2 - 4/n, 2 - 6/n etc., where n- whole numbers. But we must pay attention to the fact that in such comparisons we apply the "law" to cases that do not correspond to the examples on which it was derived, and its disagreement with observation is then nothing surprising. The "law" was established by Dalton when comparing swamp gas with ethylene and when studying nitrogen oxides, and one has only to pay attention to the modern formulas of these compounds in order to see that compounds of various series and various degrees of oxidation were compared, in a word - of various limits, but with a constant the mass of one of the elements in them; and with this restriction, the "law" is valid even now, as can be seen even in the formulas of hydrocarbons, when compared with each other, the series: C 2 H 2, C 3 H 2, C 4 H 2 ..., CH 4, C 2 H 4 , C 3 H 4 ..., C 2 H 6 , C 3 H 6 , C 4 H 6 ... etc.; in such a comparison, we find both relatively simple integers and the rule that "body masses AT, per constant body weight BUT, are in multiple ratios to each other, expressed as ratios of integers; these same examples can also serve to illustrate the circumstance that especially attracted Dalton's attention and which consists in the fact that "chemical" compounds occur in jumps; it is really clear that on H 2 has a mass of carbon equal to 24, 36, 48, H 4 - 12, 24, 36 ..., H 6 - 24, 36, 48, etc., i.e., a very small number of numbers are repeated and there is no continuity. To explain this, Dalton proposed his "atomic" hypothesis [See "Alembic Club Reprints", no. 2, 1893, "Foundations of Atomic Theory" by J. Dalton a. Wollaston (1802-1808) and Ostwald" s "Klassiker etc.", no. 3.1889: "Die Grundtagen der Atomthéorie" von J. Dalton u. W H. Wollaston (1803-08). Wed besides Art. Debus "a (l. c.) Dahem" a (l. c.) and A. Hannequin, "Essai critique sur l" hypothese des atomes dans la science contemporaine "(P. 1899)]. The concept of the atomic structure of matter is undoubtedly of very ancient origin (see Substance), but Dalton seems to have it (Roscoe a. Harden, "A New Wiew of the Origin of Daltons Atomic Theory, 1896; eg also in Zeit. f. Ch., 1896), developed under the influence of Newton, who needed atoms to build his theory of the outflow of light. Newton developed his view in the questions that ended his Optics; Thus, in Question XXXI, Newton asks: "Do not the smallest particles of bodies have certain properties, abilities or forces that allow them to influence at a distance not only the rays of light in order to reflect, refract and deflect them, but also on each other and cause in this way most natural phenomena? When two bodies are connected, Newton considers the connection as a consequence of the mutual attraction of the smallest particles of both bodies at small distances. “When potash blurs, is it not due to the mutual attraction between its particles and particles of water, rushing over them in the form of steam? particles of water? The immediate reason for the adoption of atomic views for Dalton was, it seems (erroneous, as we now know), the observation that nitric oxide can react completely with atmospheric oxygen or in relation to 36 vol. NO per 100 rpm air, or in relation to 72 vol. NO for the same 100 rpm. air, and in the first case nitrous acid is formed, and in the second nitric acid; "These facts," he says, "clearly indicate the theory of the process: the elements of oxygen can combine with a certain amount of nitric oxide, or double, but not with any of the intermediate amounts." He was brought to atomic views by the study of the solubilities of various gases in liquids, and the gas pressure in mixtures. At least, we see that no more than a year after the said experiment (September 6, 1803), he is busy "observing the final particles (ultimate particles) of bodies and their combination", and to his message " On the Absorption of Gases by Water and Other Liquids, read on 21 Oct. 1803 ("On the Absorption of Gases by Water and other Liquids", reprinted abbreviated in Ostwal's "Klassiker", see above) the first table of relative weights is attached (very inaccurate), entitled: "Table of the relative weights of the ultimate particles of ga s eous and other bodies"; in it the elements: hydrogen, nitrogen, carbon, oxygen, phosphorus, sulfur are listed interspersed with various compounds, between which there are some organic substances, and with each name a figure of relative weight is given finite particles without explaining how it was obtained by the author In 1804, he communicated his views to Professor Thomson (from Edinburgh), who visited him in Manchester, and the latter published them (with the consent of Dalton) in the 3rd volume of his textbook X. , published in 1807. Finally, in 1808 they were set forth by Dalton himself in his "A New System of Chemical Philosophy" (see Oslwald's "Klassiker" l. p.). The following passages characterize the most significant points of Dalton's views. "Such observations (we are talking about observations on the three states of bodies: gaseous, liquid and solid) led everyone to a tacit agreement that bodies of appreciable size, whether liquid or solid, consist of an enormous number of unusually small particles, or atoms, matter held together by a force of attraction, more or less significant, depending on the circumstances, we call it cohesion when it prevents particles from separating, or ... affinity when it collects them from a dispersed state (for example, when steam turns into water) ... A rather important question is whether the final (last) particles of a given substance, for example water, are the same, i.e., have the same appearance, the same weight, etc. Based on what we know, we have no reason to assume any difference between them; .. one can hardly imagine that aggregates of non-identical particles could be so homogeneous. If some of the particles of water were heavier than others, and if by chance some part of this liquid consisted mainly (?) of them, then this should affect the specific gravity of the water, which was not observed. The same considerations apply to other bodies. We must, consequently, conclude that the final particles of any homogeneous body are completely identical with each other with respect to their weight, shape, etc. In other words, each particle of water is identical with every other particle of it, each particle of hydrogen is completely identical with the other a particle of hydrogen, etc.". "One of the main tasks of this work is to indicate the importance and benefit of determining the relative weight of ultimate particles, both simple and complex bodies, the number of simple particles of an element that make up a complex particle ... If two bodies are given, BUT and b, prone to connection, then the following combinations of them are possible, starting with the simplest, namely:
1 body atom A+ 1 atom B= 1 atom WITH, binary
1 atom A+ 2 atoms AT= 1 atom D, triple
2 atoms BUT+ 1 atom B= 1 atom E, triple
1 atom A+ 3 atoms AT= 1 atom F, quadruple
3 atoms A+ 1 atom AT= 1 atom g, quadruple
etc. The following general rules may be taken as guidelines for investigations concerning chemical synthesis. 1) If only one compound can be obtained for two reacting bodies, then it must be assumed that it is binary, unless some reason forces one to speak in favor of the opposite opinion. 2) If two compounds are observed (for 2 elements), then one must think that one of them is binary and the other is triple. 3) When three compounds are known, we should expect one of them to be binary and two of them to be ternary. 4) When four compounds are known, we must expect that one of them is binary, two are ternary, one is quaternary, etc. 5) A binary compound must always be specific heavier than a simple mixture of both of its constituent bodies. 6) A ternary compound must be heavier than a mixture of a double compound with a simple one, which, when combined, could form a complex compound, etc. 7) The indicated rules and remarks are equally applicable when bodies such as With and D, D and E... From the application of these rules, we deduce the following conclusions: 1) that water is a binary compound of hydrogen and oxygen, and that the relative weights of both elementary atoms are approximately 1:7; 2) that ammonia is a binary compound of hydrogen and nitrogen, and that the relative weights of both elementary atoms stand to each other approximately as 1:5; 3) that nitric oxide is a binary compound of nitrogen and oxygen, the atoms of which weigh 5:7, respectively ... In all cases, the weights are expressed in hydrogen atoms, each of which is equal to one ... Due to the novelty, as well as the importance of developed in this chapter ideas, it has been found appropriate to give tables illustrating the method of connection in some of the simplest cases ... The elements, or atoms, of such bodies, which are currently considered elementary, are indicated by small circles with some conventional signs (see Formulas); the compound consists in the juxtaposition of two or more atoms "... At present, the complete arbitrariness of these guiding rules is involuntarily striking. It is obvious that the composition of the compound is in no way dependent on whether we know, or not, the conditions for the formation 2 elements of several compounds, and our disagreement in this respect with Dalton is best illustrated by the fact that we give water the formula H 2 O, and ammonia H 3 N, i.e. we consider the first not a binary, but a triple body, and the second - quaternary. Then, it is not clear why, in the presence of two compounds, one should be binary, and the other ternary; while for hydrogen with oxygen, two compounds are known with certainty, but we now consider one to be ternary - H 2 O, and the other quadruple - H 2 O 2 (hydrogen peroxide) It is also undoubted that position 5 is in sharp disagreement with all "substitution" reactions and, for example, with the classical reaction of the formation of hydrogen chloride:
H 2 + Cl 2 \u003d 2HCl,
when, as you know, ud. the weight of the mixture of hydrogen with chlorine is, within the accuracy of observations, sp. the weight of hydrogen chloride, etc. Meanwhile, the influence of Dalton's views on the development of X. was enormous and continues to this day; the question is, what caused it, when the very idea of the atomic structure of matter does not belong to Dalton? As far as one can judge, this influence is due to the following circumstances: 1) The discontinuity of the matter surrounding us, the lack of continuity in it affects us so much that we cannot figuratively imagine it to be continuous, and all attempts in this direction have so far turned out to be extremely difficult to understand and fruitless; it is obvious that due to the same circumstances, atomic ideas arose even among the ancients. 2) Dalton showed the practical applicability of atomic views to chemistry; having accepted that the atoms of various elements differ in relative weight [In this respect, he differed from Higgins" (1790), who believed that the basic atoms were identical to each other, and attributed all the observed differences in matter to their larger or smaller clusters. Views of Higgins "a were resurrected first by Praut" th, and now by J. J. Thomson "th]; he gave an unusually simple and easily accessible scheme, in which the existence of both compounds of constant composition and compounds subject to the law of "multiple ratios" fit with surprising ease. The clarity and applicability of the scheme in the eyes of several generations of chemists even served as an "explanation" of these laws, and only now it turns out that "constancy of composition" is possible much more often than previously thought, that the factor that determines it is the known relationship between the as yet indeterminate "nature "reacting bodies, the type of external energy acting on the system and the physical heterogeneous complexes (phases) of which it is composed. As for the law of "multiple ratios", it still does not have a generally accepted explanation; the comparison given by Wald with the law of rational parameters in crystallography is unsatisfactory due to the low visibility and insufficient clarity of the main provisions; N. S. Kurnakov agrees with Wald's view in his report "On the Fusibility of Metal Alloys" to the XI Congress Est. and vr. in St. Petersburg. in 1901; the parallelism of both propositions can hardly be questioned; but, if in crystallography the said law even has a mathematical proof, which seems to be based on the impossibility of the existence of spherical crystals, then it is still not clear what parallel position X should be taken. On the other hand, Duhem says: “It is obvious that the answer (of the atomic theory to the phenomena of multiple ratios) is satisfactory and can even be considered a victory for the atomic theory, a victory all the more noticeable because this explanation of the law of multiple ratios was not subsequently adjusted, which, on the contrary, , it is coeval with the law, and perhaps preceded its discovery. Is this victory final? In order for this to be so, it is necessary not only that the explanation of multiple relations given by atomic theory be a probable current, but also the only possible ones. But who will he dare to take upon himself the guarantee of this interpretation and dare to assert that it will never be possible to find another? word, but also the very idea of atoms [Duhem refers to the exposition given by him in the cited work ("Le mixte et la comb. chim.", 1902).]; We, if we pay attention to the contradictions that immediately arise as soon as we explain these principles from the atomic point of view [Cf. Stallo, "La Mati ère et la Physique moderne".], it is difficult to defend against the idea that the only success of the atomic theory represents an apparent victory for which tomorrow is not secured; that this theory does not acquaint us with the true, objective cause of the law of multiple ratios; that this reason must still be discovered, and finally, that modern X. does not speak in favor of the doctrine of Epicurus. "No matter how the future answers, the point is this: Dalton noticed the existence of "multiple relations" and considered that these phenomena follow from the atomic representations, because they correspond to the simplest possible combinations of atoms; now we know a huge number of systems with an indefinite composition, and not only in gaseous and liquid states, as was the case in Dalton's time, but also in solid (starting with isomorphic Mitcherlich mixtures and ending with solid fan "t Hoff solution); it cannot be said that these phenomena directly contradict the atomic structure of matter, but instead they require an explanation why they are not constantly observed, and it is obvious that we cannot rest at ease in this explanation alone. 3) Finally, Dalton's law of multiple ratios gave chemists an easily accessible criterion for judging whether they are dealing with one individual body or with a complex system formed by the interaction of two or more bodies that are stable under experimental conditions. This side of the subject was not clearly formulated by contemporaries, but the importance of the law itself did not escape their attention, and Thomson soon (Jan. medium salt, and Wollaston discovered (January 28, 1808) simple, multiple ratios for some acid, carbonic and oxalic acid salts, and then for the definitions atomic weights Berzelius is accepted and devotes several years of persistent and unusually careful work to them [Cp. Ostwald "s, "Klassiker", No. 35, "Versuch die bestimmten und einfachen Verhältnisse autzufinden, nach velchen die Bestandtheile der unorganischen Natur mit einander verbunden sind, von J. Berzelius" - 1818-19; Berzelius then gave several Here is not the place to dwell on the difficulties that chemists encountered in establishing precisely atomic weights, and how Dalton's rules were gradually eliminated, and Berzelius drew on the laws of heat capacity of solid elements, Dulong and Petit, Mitcherlich's isomorphism (1819); we confine ourselves to pointing out that all this turned out to be insufficient, and modern atomic weights were established only after the so-called " molecular theory"Avogadro-Ampera.
Volumetric laws of Gay-Lussac. Lavoisier (Oeuvres etc., I, 73 and 75) noted that in order for oxygen to combine with hydrogen to form water, it is necessary to take twice the volume of hydrogen per volume of it; this circumstance was subsequently disputed (Dalton, for example, thought that for 185 hours of hydrogen one should have 100 volumes of oxygen), and therefore it was important that A.F. Humboldt and Gay-Lussac, with extremely thorough experiments for that time, established ["Exp ériences sur les moyens endiométriques et sur la proportion des principes constituants de l" atmosphè re", 1805; see Ostwald's, "Klassiker" No. 42.] that Lavoisier was right and that, indeed, 200 rpm . hydrogen is required for the formation of water 100 vol. oxygen. At this time, there was already a dispute between Proulx and Berthollet about the constancy of the composition of chemical compounds, on the other hand, Dalton in his "New System of chemical Philosophy" spoke in favor of the invariable atomic composition of "chemical" compounds, and therefore Gay-Lussac in 1808 ( memoir "Sur la combinaison des substances gazeuses, les unes avec les autres" [See Ostw. "Klas." No. 42.] undertook a long study on the interaction of various gases, the results were favorable to the views of Proulx and Dalton, namely, Gay-Lussac found that "that the combinations of gaseous bodies with each other always occur in very simple ratios, so that 1, 2 and, at most, 3 volumes of the other are combined with one volume of one gas. These volume ratios are not observed for liquid and solid bodies, but equal to way, and for the weights of reacting bodies, which constitutes a new proof that only in the gaseous state bodies are in the same circumstances and obey the correct laws. which gases emit when combined is also in a simple ratio to the volume of one of them, and this is also characteristic of the gaseous state. Usually in modern textbooks Gay-Lussac's observations are summarized in the form of two laws: 1) The volumes of reacting bodies in the gas and vapor state are either equal or are in simple ratios expressed by ratios of simple small integers and 2) The volume of the formed body in the gas and vapor state is always in a simple ratio to the volume (gas-vapor) of each of the constituent parts included in it. Gay-Lussac's experiments seem to have ended Berthollet's dispute with Proulx. Strange as it may seem at first glance, Dalton reacted negatively to them, namely, in addition to his "New System of Chemical Philosophy" he criticizes Gay-Lussac's observations on the interaction of nitric oxide and oxygen (indeed, erroneous) and adds: "On in fact, what he says about volumes is analogous to what I say about atoms; and if it could be proved that all gases (elastic fluids) contain in equal volumes an equal number of atoms, or numbers related as 1, 2, 3, etc., then both hypotheses would coincide, except that mine is universal, and his is applicable only to gases. Gay-Lussac, however, could not fail to see that such a hypothesis was considered by me and discarded as worthless [Dalton refers to a passage in his book where he says that he once had a vague belief, which he shared with many others, that in equal volumes of any gases (simple and chemically complex) there are an equal number of atoms, but he should have given it up firstly, on the basis of observations on the interaction of oxygen with nitric oxide, when a mixture of equal volumes of gases is sometimes reduced by half, which indicates that in the final body there are fewer atoms per unit volume than in the initial ones (this observation is incorrect), and secondly, because ud. the weight of water vapor is less than sp. the weight of the oxygen that forms it, which would be impossible if it were formed by the combination of 2 hydrogen atoms (2 vol.) with 1 oxygen atom (1 vol.), but he revived this idea, and I will do a few things about him remarks, although I have no doubt that he himself will soon see the inconsistency of his view. " Dalton ends like this: "The truth, I am convinced, is that gases never combine in equal or simple ... volumes; nowhere is there a closer approximation to mathematical accuracy than in the case of hydrogen with oxygen, and meanwhile, the most accurate of my experiments show: here at 1.97 vol. hydrogen accounts for 1 vol. We now know that Gay-Lussac was undoubtedly closer to the truth than Dalton, and it was in the case of hydrogen with oxygen that Morley and Scott showed that the true ratio was 2.002 to 1.
Avogadro position. In June 1811, the Italian physicist A. Avogadro undertook to reconcile Dalton's views with Gay-Lussac's observations in an article entitled: "Essai d" une mani ère de déterminer les masses relatives des molécules élémentaires des corps, et les proportions selon lesquelles elles entrent dans le s combinaison" [The nomenclature that Avogardo follows in this article differs from ours; as J. Walker notes, his molecule = atom, molecule (it doesn't matter), mol écule inté grante = molecule (primarily complex bodies), mol é cule constituante - a molecule of an elementary body and mol écule élé mentaire - an atom of an elementary body, but one of the places in the article makes one think that mol écule inté grante also means an atom (cf. Ostwald "s," Klassiker ", No. 8).]. “Gay-Lussac showed in an interesting memoir,” writes Avogadro, “that the combinations of gaseous bodies always occur in very simple volumetric ratios and that, in the case of a gaseous reaction product, its volume is also in simple ratios to the volumes of the reacting bodies. But the ratios between the masses constituents in a compound seem to depend only on the relative number of reacting molecules (and their masses) and on the number of complex molecules formed.Therefore, it must be concluded that there are very simple relations between the volumes of gaseous bodies and the number of molecules composing them. , apparently, the only acceptable hypothesis should be recognized that the number of molecules of any gases is the same in equal volumes, or is always proportional to the volume. Indeed, if the number of molecules in equal volumes was different for different gases, then it is difficult it would be understandable that the law governing the distance of molecules leads in all cases to such a simple connection as the above, to which we are forced to recognize between the volume and the number of molecules ... Based on this hypothesis, we apparently have the means to easily determine the relative masses of molecules for bodies capable of existing in a gaseous state, as well as the relative number of molecules necessary for the reaction; namely, the ratios of the masses of molecules under this assumption are the same as the ratios between the specific gravities of different gases (at equal temperatures and pressures), and the relative number of reacting molecules is given directly by the ratio of the volumes of gases forming a given compound. For example, since the numbers 1.10359 and 0.07321 express the specific gravity of the gases of oxygen and hydrogen (the weight of an equal volume of air \u003d unit specific weights [These numbers are incorrect.], Then their ratio, otherwise, the ratio between the equal volume masses of both gases, represents, according to our hypothesis, the ratio between the masses of their molecules, from which it follows that the oxygen molecule is almost 15 times heavier than the hydrogen molecule, or, more precisely, they are related as 15.074 to 1. .. [The ratio given here is incorrect (see Chemical Formulas). To understand Avogadro's reasoning, let's denote the weight of the oxygen molecule as M, the weight of a hydrogen molecule through 1, then the weight of a certain volume of oxygen will be - xM, where x the number of oxygen molecules in this volume, and the weight of the same volume of hydrogen = x 1(by position). Known ud. weights of both gases in relation to to air, i.e. the values: (xM)/p and (x 1)/p, where R - the weight of an equal volume of air; it's obvious that [(xM)/p]:[(x 1)/p] = M/1, i.e., equal to the ratio between the weights of oxygen and hydrogen molecules, of which the latter is taken as a conventional unit of measurement.]. On the other hand, since we know that the ratio between the volumes of hydrogen and oxygen during the formation of water = 2:1, then, therefore, we know that water is formed during the interaction of each oxygen molecule with two hydrogen molecules ... But there is an argument, which, at first glance, speaks against the assumption of our hypothesis for complex bodies. It seems necessary that a complex molecule formed by the interaction of two or more molecules of simple bodies should have a mass equal to the sum the masses of these latter; or in particular, when a complex body is obtained by the interaction of 1 mol. one body with 2 or more mol. another body to the number of complex mol. remained equal to the number of first body. In the language of our hypothesis, this is equivalent to the fact that, when a gas is combined with two or more volumes of another gas, the volume of the compound in the gaseous state must be equal to the volume of the first gas. And yet, in a huge number of cases, this is not observed. For example, the volume of water in the gaseous state, as shown by Gay-Lussac, is twice the volume of oxygen used to form it, or, what is the same, equal to the volume of hydrogen, instead of being equal to the volume of oxygen. But the way of interpreting these facts in accordance with our hypothesis also presents itself; namely, we assume: 1) that the molecules of any elementary bodies ... are not formed by individual elementary molecules (atoms), but are composed of a certain number of them, connected by mutual attraction together, and 2) that when the molecules of another body are combined with the molecules of the first, forming a complex molecule, then the integral molecule, which should be formed, breaks up into two or more parts, formed from half, a quarter, etc. the number of molecules of the first body entering into combination, connected with a half, a quarter of the molecules of the second body ..., so that the number of final molecules becomes double, quadruple, etc., in comparison with what it would be without disintegration, and just such as is required by the observed volume ratio of the resulting gas ["Thus, for example, the final molecule of water must be composed from a half-molecule of oxygen combined with one molecule, or two half-molecules, of hydrogen" (approx. Avogadro). The act of connection 2 about. hydrogen with 1 vol. oxygen Avogadro imagines, next, as a compound 2x they say hydrogen from 1 x they say oxygen to form initially 1x complex mol. water containing each 2 mol. hydrogen and 1 mol. oxygen, but then decaying into 2x simpler piers, the mass of which is already
(2x mol. hydrogen + x mol. acid) / 2x = (2 mol. hydrogen) / 2 + (mol. sour) / 2 = mol. hydrogen. + (mol. sour)/2;
each volume of water vapor contains 2 times less oxygen than an equal volume of oxygen gas, in the latter it was x they say sour, and an equal volume of steam contains
x mol. water \u003d x (mol. hydrogen + mol. acid / 2).].
Reviewing the various, most well-studied, gaseous compounds, I only find examples of doubling the volume of one of the terms, connecting with two or more volumes of another body [The expression is incorrect, but, unfortunately, often used. Undoubtedly, no doubling of volume is observed here; on the contrary, its reduction occurs; Avogadro, on the other hand, speaks of doubling, because, according to his assumption, the volume of the reacting bodies is initially reduced to one volume. At present, there are much more complex examples and the equation for the formation of hydrogen sulfide at temp. boiling sulfur:
S 8 + 8H 2 \u003d 8SH 2
Avogadro should have explained by the formation of the initially complex molecule S 8 Η 16 and the subsequent octalization of its volume: S 8 H 16 \u003d 8SH 2 .]. We have already seen this for water. Similarly, we know that the volume of ammonia is twice the volume of (free) nitrogen in it. But it is possible that in other cases the molecules will be divided into 4, 8, etc. The possibility of such a division should also be expected a priori... volumes and not changing, moreover, as, for example, in the case of nitric oxide [Composition and sp. the weight of nitric oxide is given in the formula NO, the formation of which from nitrogen and oxygen can only be represented by the equation
N 2 + O 2 \u003d 2NO.
In fact, this reaction has not yet been carried out. Good examples are reactions:
H 2 + Cl 2 \u003d 2HCl,
H 2 + Br 2 \u003d 2HBr,
occurring without volume change.]. Under the hypothesis of the divisibility of molecules, it is easy to see that the combination here actually turns two kinds of molecules into one and that a reduction by at least the volume of one of the gases would have to wait if each complex molecule (see note above) was not divisible into two others, identical in nature... Based on arbitrary assumptions about the most probable number of molecules (atoms) in compounds, Dalton tried to establish relationships between the molecules of simple bodies. Our hypothesis... makes it possible to correct his data... Thus, for example, Dalton assumes that water is formed by the combination of hydrogen and oxygen molecule by molecule (atom by atom). On the basis of this, and on the basis of the relative weights of both bodies contained in water, it follows that the mass of the oxygen molecule must be related to the mass of the hydrogen molecule as 7½ to 1 approximately, or, according to Dalton himself, as 6 to 1. According to our hypothesis, this ratio is just twice as large, namely = 15:1. As for the water molecule, it should equal in round numbers 15 + 2 = 17 (taking the hydrogen molecule as 1) if it were not divisible by 2; but by virtue of this division, it becomes half as much, i.e., 8½, or, more precisely, 8.537, as can be found directly by dividing beats. weight of water vapor, i.e. 0.625 (Gay-Lussac; specific weight is given in relation to air) per sp. the weight of hydrogen is 0.0732. This mass differs from the 7 assigned by Dalton to the water molecule, only because of the difference in the numbers for the composition of water adopted by Dalton, etc. That Avogadro's views were little appreciated by his contemporaries is not surprising. Dalton could not agree with them because he generally doubted the correctness of Gay-Lussac's observations, and besides, Avogadro's views went against his convictions about the indivisibility of atoms; it is more strange that later Avogadro's article remained completely forgotten and that even now one can find many misunderstandings in textbooks on this It must be clearly seen that Avogadro's proposition: "Equal volumes of any gases at equal temperatures and pressures contain an equal number of molecules", or vice versa: "An equal number of molecules of gases taken at equal temperatures and pressures correspond to equal volumes", represents, strictly speaking, not a "hypothesis", but a purely conditional definition, and nothing more [Ostwald in his "Grundlinien" calls it Avogadro's postulate .]; by accepting it, we agree to depict our compounds in such a way that their reactions obey Gay-Lussac's laws, that is, i.e., so that each formula in the gaseous state corresponds to some conventional normal volume under normal conditions, and it is clear that we can thus express all the transformations that X. deals with, because they are all conceivable as occurring in the gaseous state ; that our formulas agree with reality not only at the temperature and pressure of experience, but also at others - stems simply from the relatively wide applicability of the laws of Boyle-Mariotte and Charles-Gay-Lussac (see Gases). When the experimental data on beat. the weights of a given vapor do not agree with the formula we expect, then we usually look for such a temperature and such a pressure at which such agreement is observed, or else we completely leave aside the experimental data and write "molecular" formulas that do not correspond to Avogadro's "law"; so, in any organic X. you can find that the molecule of acetic acid. has the formula: C 2 H 3 O (OH), that the existence of 3 hydrogen atoms in acetic acid, not in the form of an aqueous residue, is clear from the fact that, when the acid is treated with chlorine, we can successively replace 1/3, 2 / 3 and, finally, 3/3, i.e. all hydrogen is chlorine; meanwhile, there is no doubt that at a temp. boiling, the formula of acetic acid vapor corresponds closely - C 4 H 8 O 4, and the formula of monochloroacetic acid is closer to C 4 H 6 Cl 2 O 4 than to C 2 H 3 ClO 2. Many more such examples could be cited, but even the one cited already shows quite clearly that we are not dealing with "Avogadro's law", i.e., not with such a numerical ratio, which is objective and which does not depend on our arbitrariness, but with a way of expressing , calculation of experimental data. It is possible that the actual number of molecules contained in a given volume of some gas (unless the molecules represent our fiction) has nothing to do with the number of molecules established by Avogadro's proposition, and it is conceivable that in equal volumes of two gases (at equal temperatures and pressures) is in fact a completely different number of them [Since the law of Boyle and Charles - PV = RT is not mathematically accurate, then, even considering Avogadro's position to be strictly consistent with reality, we must admit that the mathematical equality of molecules in equal volumes of two gases is possible only at a certain specific temperature point and at a certain specific pressure (or with some specific and artificial ratios between the masses of gases and the volumes occupied by them).]; Gay-Lussac's laws, which are found empirically and are completely independent of our ideas about the structure of matter, such an assumption will not affect in the least: they will remain as inexplicable as the "law of multiple" ratios, which they represent for gaseous bodies, is inexplicable. It is very unfortunate because in some textbooks X. you can find a mathematical proof of the accuracy of the "law", and moreover, the proof initiated by Maxwell ("Theor y of Heat", L., 1894, 325; "Law of Gay-Lussac") . “Consider,” he says, “the case where two gases are in thermal equilibrium. We have already shown that if Μ 1 and M 2 represent the masses of individual molecules of these gases, a V 1 , and V 2 velocities of agitation corresponding to them, it is necessary that, according to equation (1), at thermal equilibrium
M 1 V 1 2 = M 2 V 2 2 .
If the pressures of both gases p 1 and p 2 and the number of molecules per unit volume N 1 and N2, then according to equation (2)
p 1 = 1/3 M 1 N 1 V 1 2
R 2 = 1/3 M 2 N 2 V 2 2 ;
if the pressures are equal, then
M 1 N 1 V 1 2 \u003d M 2 N 2 V 2 2,
and if the temperatures are equal, then
M 1 V 1 2 = M 2 V 2 2 ;
dividing the last two equations term by term, we find that Ν 1 = N 2(6), or that when two gases are at the same temperature and the same pressure, then the number of molecules per unit volume is the same for both gases. "It seems obvious to the writer that even when the pressures of two different gases are equal , which are at thermal equilibrium, the expressions for R 1 and R 2 cannot be equated until it has been proven that this must imply equal volumes of both gases; this is assumed by Maxwell, since N 1 and N 2 refers to them as "units of volume", but the need for such an assumption cannot be considered obvious, because the pressure of the gas, once established, has no relation to the volume occupied by the gas. Thanks to this arbitrary choice, an indefinite problem in itself acquired a definite solution. Clausius (1857) was more cautious in this respect; he assumed that in equal volumes of gases there is an equal number of molecules, and already from this he deduced with the help of the kinetic theory of gases that their living forces must be equal. Thus, we cannot have a proof of Avogadro's proposition, but it is certain that once we accept his definition, we will be able to easily establish the relative weights of molecules (the relative weights of equal volumes of gases); the whole thing boils down to two definitions of beats. weights of the gases being compared, and, as we saw above, it is completely indifferent in relation to which gas the sp. weight. Avogadro considered the hydrogen molecule to be the unit of molecular weights (see above); now very often such a unit is considered to be a hydrogen atom. The next question is how many hydrogen atoms are in its molecule and what definition of the word "atom" can be given, following the terminology of Avogadro. It has been found by experience that chemical interaction gaseous bodies, often one of them after the transformation is in a larger volume than before the experiment; so, for example, it was indicated above that a given mass of oxygen in the form of water vapor occupies twice the volume than the same mass of pure oxygen taken under the same conditions of temperature and pressure; together with Avogadro, we express this by saying that in the formation of water the oxygen molecule is divided into two absolutely identical halves, and therefore we recognize that chemical reactions can be accompanied by the division of molecules; experience shows, moreover, that this division often goes so far that it is inaccessible to us in any other way; so, for example, if we remain with the example just mentioned, no matter how high temperatures we compare water vapor with oxygen, there will always be twice as much oxygen in a given volume of oxygen gas as it will be contained in an equal volume of water vapor. On the other hand, the word "atom", derived from gr. sl. άτομος - indivisible, forces us to designate by it such a mass of matter that we can recognize as incapable of further simplification by division. Hence the modern definition of an atom: it is - the smallest mass of a given element with which it enters into the composition of chemically complex molecules, i.e., the molecules of such bodies in which, in addition to this element, there is at least one other element. To solve the above question, it is necessary, next, to determine the ud. hydrogen weights of various hydrogen compounds, determine by analysis what proportion of these sp. weights, expressed in hydrogen molecules, fall on hydrogen and take the smallest one for its atom; according to the Gay-Lussac law, the ratio between the mass found and the mass of the hydrogen molecule must be expressed as a simple, i.e., relatively small integer. You can do otherwise; one can compare the volumes of gaseous compounds with the volume of hydrogen contained in them; the ratio, expressed as the largest integer, gives us a measure of the divisibility of the hydrogen molecule. For clarification, let's take, as examples, hydrogen compounds: swamp gas (a compound of carbon and hydrogen), ammonia (a compound of nitrogen and hydrogen), water (a compound of oxygen and hydrogen) and hydrogen chloride (the elemental composition is given by the name itself); beats hydrogen weight of the first = 8, i.e. weight x they say swamp gas: weight x they say hydrogen \u003d 8, from where they say. swamp gas = by weight 8 mol. hydrogen; analysis shows that ¼ of this amount falls on hydrogen, next, mol. swamp gas consists of carbon (weighing 6 mol. hydrogen) and 2 mol. hydrogen; beats ammonia weight = 8½, and 1½, wt. units of this amount fall to the share of hydrogen; next, arguing in the previous way, we come to the conclusion that 1 mol. ammonia consists of nitrogen (weighing 7 mol. hydrogen) and 1½ = 3/2 mol. hydrogen; the composition of the water molecule is oxygen (in the amount = 8 mol. hydrogen) and 1 mol. hydrogen; finally, ud. weight of hydrogen chloride = 18.25, of which only 0.5 is hydrogen; next, the hydrogen chloride molecule consists of chlorine (= 17.75 mol. hydrogen) and ½ mol. hydrogen; the last value is the smallest of those found by us; consequently, we can assume that the hydrogen molecule is divisible in half, and this half can provisionally be taken as the "atomic weight" of hydrogen. Understandably, consideration of these compounds from the perspective of their bulk composition also leads to the same conclusion; the figures given above say exactly that 1 vol. swamp gas is equal to ½ vol. the hydrogen in it, 1 vol. ammonia = 2/3 vol. hydrogen contained in it, 1 vol. water vapor = 1 vol. hydrogen, available in it, and finally, 1 vol. hydrogen chloride is twice the volume of hydrogen in it; the greatest increase occurred in the formation of hydrogen chloride, and, according to Avogadro, we must recognize that the hydrogen molecule is divisible in half. Numerous determinations of the composition of a wide variety of compounds have shown that there are no chemically complex compounds in the molecule of which there would be less than half a hydrogen molecule; we can, therefore, finally call this quantity the hydrogen atom [Compare, however, the experiments of J. J. Thomson.] and, denoting it by the letter h, write the hydrogen molecule H 2 . In order to find ud. weight of gas in relation to hydrogen, we must take the ratio between the weights of equal volumes of gas and hydrogen (at a certain temperature and pressure), containing, by definition, an equal number of molecules, and therefore this sp. the weight
D \u003d (xM) / (xH 2),
where x- unknown to us the number of molecules of both gases, M is the weight of a given gas molecule, and H 2 - the weight of a hydrogen molecule, or in words: the molecular weight of a gas is D once taken the molecular weight of hydrogen; when we express it in hydrogen atoms (in halves of a hydrogen molecule), then it is equal to 2D times the atomic weight of hydrogen. Usually the latter is taken as a unit of measurement; then
M=2D,
but it must be remembered that in this expression D is an abstract number, and 2 is named, since it stands instead of 2 hydrogen atoms, and it has already been indicated earlier (see Formulas) that in the case when we consider oxygen \u003d 16, then the atomic weight of hydrogen \u003d 1.008, and so on., then
M" \u003d 2 1.008D,
where M" represents a formula in which all atomic weights are referred to O = 16, a D beats weight of steam (gas) by hydrogen. About the volume of gram molecules at H 2 = 2 and O 2 = 32 - see Formulas of chem. In conclusion, it must be pointed out that, in addition to Avogadro, they wrote on the same issue: Ampère ("Ann. de chim." 90, 1814, German translation, in Ostwald's "Klassik.", No. 8), Godin (Gandin , "Ann. chim. phys.", 35, 1833: "Recherches sur la structure intime dos corps inorganiques dé finis etc." equal volumes of gases in equal squares - a mnemonic device that Hoffmann subsequently introduced.], Gerard (see Unitary system) and, especially, Cannizzaro (St. Cannizzaro, "Nuovo Cimento", 7, 1858: "Sunto di un corso di filosofi a chimica fatto nella Reale Universita di Genova "; in German in Ostwald's "Klassiker", No. 30), who rediscovered Avogadro. All objections to the "law, Avogadro" cannot even be listed here. It is enough, as an example of misunderstandings, to indicate that the specific weight of ammonia vapor in relation to hydrogen turned out to be equal not to half of the formula, but to a quarter of it, i.e.
NH 4 Cl / 4 \u003d NH 4 Cl / 2H 2,
whence it followed that the hydrogen molecule corresponds
NH 4 Cl / 2 \u003d N / 2 + H 4 / 2 + Cl / 2;
since under the conditions of evaporation of NH 4 Cl it was impossible to allow the division of "atoms" of nitrogen and chlorine, i.e., changes in these elements, G. St. Clair Deville considered the abnormal density of the vapor of NH 4 Cl to be evidence of the inaccuracy of "Avogadro's law". S. Cannizzaro the first [Cf. E. Mitscherlich, "Ueber das Verh ältniss des spec. Gewichts de r Gasarten zu den chem. Proportionen", "Ann. Ch. Ph.", 12, 1834 and "Gesamm. Abhandl.".] indicated that disagreement could be explained by the decay of NH 4 Cl into NH 3 and HCl, which should occupy the volume of 2 "molecules" of hydrogen. Pebal's direct experience subsequently confirmed this consideration. It should be noted that in many cases of abnormal beats. There is still no experimental study of the resulting products, and therefore it may be that the interpretation now accepted will later turn out to be incorrect. So, for example, a decrease with an increase in temperature sp. the weight of a pair of acetic acid, reaching C 4 H 8 O 4 /2H 2, is usually explained by the expression:
but the following reaction is conceivable:
(acetic anhydride) + H 2 O, etc. All modern atomic weights are derived in accordance with Avogadro's definition, and therefore all modern chem. eq. (especially for gaseous bodies) can serve as illustrations of Gay-Lussac's volumetric laws.
Other laws that serve to determine the weights of molecules, atoms and equivalents. Not all compounds and elements are able to go into gaseous state. We are deprived of the possibility in such cases to establish the relative weight of the molecule in beats. vapor weight (see Determination of vapor density) and, therefore, we cannot directly determine the atomic (lowest) weight with which a given element is part of the molecules of these bodies. The last value can, however, be established indirectly in such cases, using some properties of solutions (see Solutions, Cryoscopy and Ebulioskopiya) or on the basis of isomorphism (see); we can establish the value of the atomic weight using the Dulong and Petit law or the periodic law of D. I. Mendeleev (see. Periodic Law and Weights of atoms); finally, the value of the equivalent can be established using Faraday's electrolytic law (see Electrolysis and Electrolytic dissociation). - On the quantitative laws that govern chemical transformations, the law of mass action and the fan "t Hoff law - see Chemical affinity, Chemical equilibrium, Reversibility of chemical reactions.
The history of the development of chemical views, in addition to this article, was repeatedly touched upon in this Dictionary. See: Alchemy, Substance, Air, Weights of atoms, Glycols, Glycerin, Dualism, Substitution, Isomerism, Acids, Metals and metalloids, Lactic acid, Chemical reversibility. reactions, Paraffins, Periodic law of chemical elements, Limit organic acids, Pseudomerism, Radicals, Salt, Stereochemistry, Thermochemistry, Acetic acid. (structure), Unitary system, Phlogiston, Chemical formulas, Chemical nomenclature, Chemical structure, Chemical affinity, chemical types theory, Electrochemistry, Electrolysis, Electrolytic dissociation, Ethyl, Etherene theory, Nuclear theory and biographies of all prominent chemists. Historical information about the elements and the main chemical compounds- see special articles dedicated to them.
A. I. Gorbov. Δ.