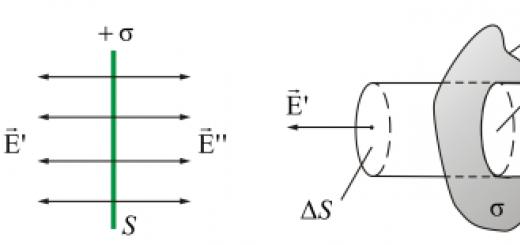
Water makes up 70-80% of the mass of living organisms.
The structure of the molecule: the electron density is shifted to oxygen, it has a partial negative charge, on hydrogen it has a partial positive charge, the molecule is a dipole. Hydrogen bonds can form between + and -.
Functions of water
1. Thanks to small dipole molecules, water is the best solvent for polar (hydrophilic) substances. In the dissolved state, substances react very quickly with each other.
2. Transport function: in the dissolved state, substances move around the body.
3. Substances on the surface of which there are no full or partial charges (hydrophobic) cannot interact with water molecules, water pushes them out (fat, gasoline). This is the basis of the structure and work biological membranes.
4. Water has an abnormally high heat capacity(it can absorb a lot of heat and still not get very hot). Due to this, it protects the cell from sudden changes in temperature.
5. Water, like all liquids, incompressible, provides support for cells (turgor) and whole organisms (hydroskeleton).
6. Water itself can participate in chemical reactions as reagent(reactions of hydrolysis, photosynthesis, etc.).
Water - the most unique substance, the basis of all living organisms on the planet. It can take various forms and be in three states. What are the main physical and Chemical properties water? It is about them that we will discuss in our article.
Water is...
Water is the most abundant on our planet inorganic compound. The physical and chemical properties of water are determined by the composition of its molecules.
Thus, the structure of a water molecule contains two hydrogen atoms (H) and one oxygen atom (O). Under normal environmental conditions, it is a tasteless, odorless and colorless liquid. Water can also be in other states: in the form of steam or in the form of ice.
More than 70% of our planet is covered by water. Moreover, about 97% falls on the seas and oceans, so most of it is not suitable for human consumption. What are the main chemical properties drinking water- you will learn further.
Water in nature and human life
Water is an essential component of any living organism. In particular, the human body, as is known, consists of more than 70% of water. Moreover, scientists suggest that it was in this environment that life on Earth originated.

Water is contained (in the form of water vapor or droplets) in different layers of the atmosphere. It comes to the earth's surface from the atmosphere in the form of rain or other precipitation (snow, dew, hail, hoarfrost) through condensation processes.
Water is the object of research for a number of scientific disciplines. Among them are hydrology, hydrography, hydrogeology, limnology, glaciology, oceanology and others. All these sciences, one way or another, study the physical and chemical properties of water.
Water is actively used by man in his economic activities, in particular:
- for growing crops;
- in industry (as a solvent);
- in the energy sector (as a coolant);
- to extinguish fires;
- in cooking;
- in pharmacy and so on.
Of course, in order to effectively use this substance in economic activity, the chemical properties of water should be studied in detail.
Varieties of water
As mentioned above, water in nature can be in three states: liquid (actually, water), solid (ice crystals) and gaseous (steam). It can also take on any form.

There are several types of water. So, depending on the content of Ca and Na cations, water can be:
- hard;
- soft.
- fresh;
- mineral;
- brackish.
In esotericism and some religions there is water:
- dead;
- live;
- saint.
In chemistry, there are also such concepts as distilled and deionized water.
The formula of water and its biological significance
Hydrogen oxide is what chemists call this substance. The formula for water is: H 2 O. It means that this compound consists of one oxygen atom and two hydrogen atoms.

The unique chemical properties of water determined its exceptional role for the life of living organisms. It is thanks to water that biological life exists on our planet.
The most unique feature of water is that it perfectly dissolves great amount other substances (both organic and inorganic origin). An important consequence of this feature is that all chemical reactions in living organisms proceed fairly quickly.
In addition, due to the unique properties of water, it is in a liquid state, with an extremely wide temperature range.
Physical properties of water
Thanks to unique hydrogen bonds, water, under standard environmental conditions, is in a liquid state. This explains the extremely high boiling point of water. If the molecules of a substance were not bound by these hydrogen bonds, then the water would boil at +80 degrees, and freeze - as much as -100 degrees.
Water boils at +100 degrees Celsius, and freezes at zero degrees. True, under certain, specific conditions, it can begin to freeze even at positive temperatures. When water freezes, it expands in volume (due to a decrease in density). By the way, this is almost the only substance in nature that has a similar physical property. In addition to water, only bismuth, antimony, germanium and gallium expand upon freezing.
The substance is also characterized by high viscosity, as well as a rather strong surface tension. Water is an excellent solvent for polar substances. You should also know that water conducts electricity through itself very well. This feature is explained by the fact that in water there is almost always a large number of salt ions dissolved in it.
Chemical properties of water (grade 8)
Water molecules have extremely high polarity. Therefore, this substance in reality consists not only of simple molecules type H 2 O, but also from complex aggregates (formula - (H 2 O) n).

Chemically, water is very active, it reacts with many other substances, even at ordinary temperatures. When interacting with oxides of alkali and alkaline earth metals, it forms bases.
Water is also capable of dissolving a wide range of chemical substances- salts, acids, bases, some gases. For this property, it is often called a universal solvent. All substances, depending on whether they dissolve in water or not, are usually divided into two groups:
- hydrophilic (dissolves well in water) - salts, acids, oxygen, carbon dioxide etc.;
- hydrophobic (poorly soluble in water) - fats and oils.
Water also enters into chemical reactions with some metals (for example, with sodium), and also takes part in the process of plant photosynthesis.
Finally...
Water is the most abundant inorganic substance on our planet. It is found almost everywhere earth's surface and in its bowels, in the mantle and in rocks, in the high layers of the atmosphere and even in space.
The chemical properties of water are determined by its chemical composition. It belongs to the group of chemical active substances. With many substances, water enters into
Water is the basis of life for all living beings. It plays an important role in the life and development of organisms:
- water is the basis of the bodies of living organisms;
- water is a medium and a participant of bio-organisms going in the bodies chemical reactions;
- water is a medium in which organisms receive many of the substances they need and get rid of metabolic products (slags);
- in plants, water is involved in photosynthesis - it consumes 5% of all the water they consume, and 95% of it goes to transpiration (evaporation by leaves, which creates an upward flow of mineral salts) and maintaining tissue turgor (elasticity);
- water is the medium of life aquatic organisms;
- the high heat capacity of water allows warm-blooded animals to maintain a constant temperature of their bodies;
- slow heating and slow cooling of water soften temperature fluctuations, which is why the climate of the coasts is called "mild", or maritime;
- the high temperature of water evaporation enables organisms to get rid of excess heat;
– other important functions.
Due to the importance of the biological functions of water, it is very often a limiting factor and, along with temperature and soil composition, determines the types of ecosystems (steppes, savannas, dry forests, moist forests).
The greatest amount of precipitation falls in the tropical zone. This is due to the maximum flow of solar energy there. Due to the high temperature, tropical air absorbs much more water than cool air at higher latitudes. Thus, the humid climate of the tropics is due to a large amount of solar energy.
The amount of precipitation is influenced by the ratio of land and sea areas: in southern hemisphere, where the area of the oceans is larger and the area of the continents is smaller, there is more precipitation than in the North.
Of great importance is not only the total amount of precipitation falling on the ground, but also their intensity and distribution over time.
Very heavy rains, especially in the absence of vegetation cover, cause soil erosion, the death of plant seedlings and small animals. Precipitation in the form of hail, the particle size of which can be the size of a chicken egg, has the strongest damaging effect. Long periods of drizzling rain are unfavorable for insects and insectivorous birds, especially during the feeding period of their chicks. In the absence of precipitation, organisms have to endure long periods of drought.
In the tropical zone, the precipitation regime is a factor that determines the seasonal activity of organisms - their biological rhythms. In temperate latitudes, the main signals of the change of seasons of the year are the duration of the daylight hours (photoperiod) and the temperature regime.
Air humidity
The indicator of air humidity characterizes the degree of its saturation with water vapor.
absolute humidity air is called the amount of water vapor per unit of its mass, and relative - the ratio of the amount of available water vapor to the maximum possible at a given temperature (in%).
Humidity is of great ecological importance.
The intensity of its evaporation from the surfaces of organisms depends on the amount of moisture in the air. At low humidity, evaporation is very strong and can lead to dehydration(dehydration) of organisms. To protect against dehydration, many of them have acquired special adaptations:
- plants - a thick cuticle, the ability to shed leaves in the dry season, the ability to fold leaves, loss (reduction) of leaves, pubescence and waxy coating on leaves, stomata immersed in leaf tissue - holes through which water evaporates;
- animals - horny scales, chitinous covers, etc.
The drying properties of air depend on deficit its saturation with water vapor - the difference between absolute and maximum possible humidity at a given temperature.
Adaptations of organisms to different levels of moisture
Plant adaptations. Depending on the need for water, all plants are divided into three ecological groups.
1. hydrophytes(from the Greek hydor - water, moisture) - moisture-loving plants, they are:
- plants that are completely in the water - elodea;
- plants in which only the roots are immersed in water - reeds, cattail, sedges, papyrus;
- plants growing in humid places - mosses, ferns, club mosses, etc.
2. Mesophytes(from Greek mesos - medium, intermediate) - plants of moderately humid places (fields, forests, meadows) have devices for obtaining water - a developed root system, integumentary and conductive tissues, and mechanisms for regulating the level of evaporation.
3. Xerophytes(from the Greek xeros - dry) - plants of dry places (dry steppes, savannas, semi-deserts, deserts) are able to tolerate a lack of moisture.
Xerophytes overcome the lack of moisture in the following ways:
- increase its absorption with the help of powerful development of root systems: in some desert plants, the mass of roots exceeds the mass of terrestrial organs by 9-10 times;
- reduce water loss by reducing evaporation from the leaves;
- accumulate water in fleshy stems (cacti and African spurges) or in leaves (aloe, agave);
- develop mechanisms to tolerate the lack of water.
Plants that accumulate water in fleshy stems or leaves are called stem and leaf succulents (from Latin succulentus - juicy). To protect against evaporation, they have a thick integumentary tissue, and cacti have stomata (holes through which evaporation occurs), deeply immersed in the leaf tissue and opening only at night, when the air temperature drops. At the same time, the root systems of succulents are poorly developed, since they grow in areas with rare but heavy rainfall.
Plants that do not accumulate moisture, but extract it from great depths and have a structure to minimize evaporation as much as possible, are called sclerophytes (from the Greek skleros - hard, hard). Sclerophytes have tough dry stems, small tough leaves that are often shed during the dry season. In many sclerophytes, the leaves are reduced (saxaul) or are spines.
Animal adaptations. There are three types of animal adaptation to drought.
1. Behavioral– migration to places where there is water, visits to watering places, nocturnal lifestyle, shelter in burrows.
2. Morphological- the presence of protective covers.
3. Physiological:
- the presence of mechanisms for the reabsorption of water in the digestive and excretory systems;
- excretion of highly concentrated or solid urine;
– synthesis of metabolic water;
- ability to tolerate severe dehydration.
List of main literature
1. Chebyshev N.V., Filippova A.V. Fundamentals of ecology. – Moscow, 2004
2.National status report environment in the Republic of Kazakhstan, MEP RK, Almaty, 2007
3. V. G. Ignatov, A. V. Kokin. Ecology and economics of environmental management., R-on-D, 2003
4. L.I. Gubareva, O.M. Mizireva, T.M. Churilova. Human ecology. M., 2005
5. G.S.Ospanova, G.T.Bozshataeva. Ecology. - Almaty, 2002
6. Edited by A.S. Stepanovskikh. General ecology. M., 2001
Water is physiologically necessary for the cytoplasm of any cell, therefore it is limiting factor both for terrestrial organisms and for those living in water, if in the latter case its amount is subject to sharp changes (ebb and flow) or it is lost by the body in very salty water by osmosis.
In the ground-air environment, this abiotic factor is characterized by the amount of precipitation, humidity, drying properties of the air, and the available area of water reserves.
The amount of precipitation depends on physical and geographical conditions and is unevenly distributed around the globe. For organisms, the most important limiting factor is the distribution of precipitation by season. In temperate latitudes, even with a sufficient amount of total annual precipitation, their uneven distribution can lead to the death of plants from drought or, conversely, from waterlogging. In the tropical zone, organisms have to experience wet and dry seasons that regulate their seasonal activity at a temperature that is almost constant throughout the year.
Air humidity It is usually measured in terms of relative humidity (the percentage of the actual water vapor pressure to the saturated vapor pressure at the same temperature). The amount of humidity affects temperature effects: lowering the humidity below a certain limit at a given temperature leads to a drying effect of the air.
The drying effect of air is most important for plants. The vast majority of plants absorb water from the soil through the root system. Dry soil makes absorption difficult. Plants adapt to the drying of the soil by increasing the suction power and the active surface of the root system.
Water is consumed for photosynthesis, about 0.5% of water is absorbed by cells, and 97 - 99% of it is spent for transpiration - evaporation of water through foliage. With sufficient water and nutrients, plant growth is proportional to transpiration. The main form of plant adaptation to soil desiccation is not a decrease in transpiration, but the cessation of growth during the drought period.
Depending on the ways in which plants adapt to humidity, there are several environmental groups, For example: hygrophytes- land plants living in very wet soils and in conditions of high humidity (rice), mesophytes- plants that can tolerate a slight drought (woody plants of various climatic zones, herbaceous plants of oak forests, etc.), xerophytes- plants of dry steppes and deserts. Xerophytes, in turn, are divided into succulents- plants capable of accumulating moisture in fleshy leaves and stems (aloe, cacti), and sclerophytes- plants with a high suction capacity of the root system and the ability to reduce transpiration due to narrow small leaves.
There is a phenomenon among succulents convergence- plants related to different types, have almost the same shape: African spurge and cactus are spherical in shape, providing a minimum evaporation surface.
Among animals, in relation to water, they distinguish their environmental groups: hygrophiles(moisture-loving), mesophiles- intermediate group and xerophiles(dry-loving). Ways of regulation of water balance in animals are divided into behavioral, morphological and physiological.
To behavioral ways include migration to more humid places, periodic visits to a watering place, the transition to a nocturnal lifestyle, etc. morphological ways of adaptation- devices that retain water in the body: shells of land snails, horny covers in reptiles, etc. physiological adaptations provide education metabolic water, which is the result of metabolism and allows the body to do without drinking water. The latter method of adaptation is used by such animals as camels, sheep, dogs, which withstand water losses in significant quantities (camels - up to 27%). A person dies already with a 10% loss of water. Poikilothermic animals tolerate water loss better because they do not have to use water to cool the body, as homeothermic animals do.
The city of Ozersk, lakes Irtyash, Bolshaya Nanoga and Malaya Nanoga located on the territory of ZATO are part of the Irtyashsko-Kasli lake system. The only drinking source of the city of Ozersk is Lake Irtyash, which is directly connected with Lake Bolshaya Nanoga. It is the lowest in the chain of lakes of the Irtyash-Kasli system, which significantly affects chemical composition water. The influence of Lake B. Nanoga is especially noticeable. Changes in the water quality of the lake. B. Nanoga entails a change in the water of Lake Irtyash.
The chemical composition of lakes Bolshaya Nanoga and Irtyash has deteriorated over the past 30 years, while Lake Malaya Nanoga has remained unchanged. Even 30 years ago, the chemical composition of lakes B. Nanoga and M. Nanoga was almost identical, now it is clear that in the water of Lake B. Nanoga the concentrations are: phosphate - an ion by 48.5 times Sulfate - an ion by 33.4 times, chloride - an ion 2.9 times, ammonium nitrogen 3.47 times higher than in the water of Lake M. Nanoga. And when the amount of foreign substances contained in it, especially those that have an adverse effect on humans, animals and plants, reaches critical values, water turns from good into evil. At present, Lake B. Nanoga has lost its significance as a fishery and drinking water body. The quality of the water in it does not meet the requirements even for reservoirs of cultural and household purposes.
The deterioration of water quality is associated with the anthropogenic factor. Every year the number of gardens in the water protection zone of the lake increases. Nutrients, phosphates, and nitrogen-containing substances enter the lake with storm and melt runoff. As a result, there is a mass reproduction of phytoplankton, primarily blue-green, green and red algae, as well as an intensive development of higher algae, which leads to a decrease in the oxygen content in the water.
Water, hydrogen oxide, H20, simplest stable under normal conditions chemical compound hydrogen with oxygen (11.19% hydrogen and 88.81% oxygen by mass), molecular weight 18.0160; colorless liquid, odorless and tasteless (in thick layers it has a bluish color). Water plays a vital role in geological history Earth and the emergence of life, in the formation of the physical and chemical environment, climate and weather on our planet. Living organisms cannot exist without water. Water is an essential component of almost all technological processes - both agricultural and industrial production.
Water is the most important component of all ecosystems, not only water, but also terrestrial, so the presence of water is an indispensable condition for maintaining ecological balance and biodiversity both in water bodies and on land.
Water is an important component of living matter. In the body of an adult animal, its content is approximately 55-65%, and in newborns - 70-80%. Water, as a universal solvent, forms dispersed, molecularly dispersed and colloidal dispersed solutions (sols and gels in tissues). These properties of water are explained by the dipole structure of its molecule, and, consequently, by the high value of the dielectric constant. Water is not only a medium for the occurrence of various chemical reactions, but also participates in the reactions of hydrolysis, hydration and dehydration, oxidation, and in some synthetic processes. The rate of hydrolytic reactions in tissues depends on the water content in tissues.
Water has a high heat capacity and thermal conductivity, due to which it is active in the thermoregulation of the animal body. Water, having good fluidity, is able to move quickly in the body; wetting the rubbing surfaces in the tissues, it improves gliding in the joints and other moving parts of the body.
The uniqueness and value of water is constantly being tested. Mankind severely attacks water and it, showing its mood, changes everything on earth, in the form of cyclones, hail, fogs, storms, hurricanes, typhoons. Quantity natural disasters increases annually. Over the past 30 years, 4 million people have died because of them, and about 4 billion have suffered.
Biogeochemical properties heavy metals
Heavy metals are elements of the periodic table with a relative molecular weight more than 40. It so happened that the terms "heavy metals" and "toxic metals" have become synonymous. To date, cadmium, mercury, lead, antimony are unconditionally classified as toxic. The activity of a significant part of the rest in living organisms can only be assessed as "excellent". Indeed, metals in ionic form are part of vitamins, hormones, regulate the activity of enzymes. It has been established that Mo, Fe, V, Co, W, B, Mn, Zn are necessary for protein, carbohydrate and fat metabolism; Mg, Fe, Cu, Zn, Mn, Co are involved in protein synthesis; in hematopoiesis - Co, Cu, Mn, Ni, Zn; in breath - Mg, Fe, Cu, Zn, Mn, Co. It is true that there are no harmful substances, there are harmful concentrations. Therefore, ions of copper, cobalt or even chromium, if their content in a living organism does not exceed the natural one, can be called microelements, but if they are genealogically associated with a factory pipe, then these are already heavy metals. Heavy metals (mercury, lead, cadmium, zinc, copper, arsenic) are among the common and highly toxic pollutants. They are widely used in various industrial productions, therefore, despite the treatment measures, the content of heavy metal compounds in industrial sewage pretty high. Large masses of these compounds enter the ocean through the atmosphere. Mercury, lead and cadmium are the most dangerous for marine biocenoses. Mercury is transported to the ocean with continental runoff and through the atmosphere.
According to one classification, more than 40 elements with a high relative atomic mass and a relative density greater than 6. According to another classification, this group includes non-ferrous metals with a density greater than that of iron (lead, copper, zinc, nickel, cadmium, cobalt, tin, antimony, bismuth, mercury).
According to the information presented in the Handbook of Elementary Chemistry, ed. A. T. Pilipenko (1977), elements with a density of more than 5 g/cm3 are classified as heavy metals. Based on this indicator, 43 out of 84 metals should be considered heavy. Periodic system elements. Among these 43 metals, 10 have, along with metallic properties, signs of non-metals (representatives of the main subgroups VI, V, IV, III of groups of the Periodic system, which are p-elements), so the term " heavy elements", but in this publication we will use the term "heavy metals" generally accepted in the literature.
Thus, more than 40 chemical elements with a relative density of more than 6. The number of hazardous pollutants, given the toxicity, persistence and ability to accumulate in the environment, as well as the extent of distribution of these metals, is much less.
First of all, of interest are those metals that are most widely and in significant volumes used in production activities and, as a result of accumulation in the external environment, pose a serious danger in terms of their biological activity and toxic properties. These include lead, cadmium, zinc, cobalt, nickel, copper, manganese.
In aqueous media, metals are present in three forms: suspended particles, colloidal particles, and dissolved compounds. The latter are represented by free ions and soluble complex compounds with organic (humic and fulvic acids) and inorganic (halides, sulfates, phosphates, carbonates) ligands. Big influence the content of these elements in water is affected by hydrolysis, which largely determines the form of the presence of the element in aqueous media. Much of the heavy metals are transported surface waters in a balanced state.
Sorption of heavy metals by bottom sediments depends on the characteristics of the composition of the latter and the content organic matter. Ultimately, heavy metals in aquatic ecosystems are concentrated in bottom sediments and biota.
Material and Method
The samples of the water of the lake and two species of fish living in it: perch and whitefish were subjected to a study on the content of heavy metals. The UGAVM laboratory determined the content of: copper, iron, cobalt, nickel, lead, zinc, cadmium, manganese, magnesium.
It turned out that in the water of the lake for a number of elements the excess of MPC is expressed: copper by 56 times, zinc by 16 times, nickel by 4 times and manganese by 2 times, the iron content was at the upper level of MPC.
The results of a study of nine heavy metals in the tissues of fish living in Lake Bolshaya Nanaga indicate that their levels for the most part do not exceed the MPC.
At systems approach Based on these results, it was established that the fish organism forms a two-tier pyramid.
At its first level there are two subsystems, the first of which contained three elements. Its activation is caused by a change in the content of iron in the tissues of fish, the result of the activity of the subsystem was a significant decrease in cobalt.
The subsystem of the second order contained three elements. Activation occurred due to changes in the content of zinc in fish tissues, the result of the activity was the desire to reduce cadmium.
At the second echelon, one subsystem was formed by the fish organism. The element of its activation was iron, the result of the activity was a significant decrease in zinc.
Cadmium turned out to be outside the subsystem, due to the lack of control mechanisms.
Thus, if the state of the water indicates a significant excess of the MPC of four elements out of nine (copper, zinc, nickel and manganese), in the body of fish there are also four, but somewhat different (cadmium, lead, nickel manganese), although the MPC for fish tissues is not exceeded the norm.