There is a lot of water in living organisms. In most cases, it makes up more than half of the mass of a living organism, and sometimes its share in the body is 95-99%. All this is due to the extremely important role of water for the life of living organisms. And this value is due to the special properties of water, which it owes to its structure.
The water molecule consists of two hydrogen atoms and one oxygen atom. These atoms form the polar poles of the molecule (the positive pole is the hydrogen atoms, and the negative pole is the oxygen atom). The existence of poles makes possible the formation of hydrogen bonds, which allow water molecules to form various complexes with each other and with other substances. Such complexes of molecules significantly increase the boiling and melting points of water (compared to similar molecules) and increase its heat capacity. They also make water a very good solvent and a favorable environment for a number of reactions to take place.
The most important properties of water for living organisms are the following:
1. Water is an excellent solvent for polar substances and non-polar substances with charged sites.
2. Water is capable of forming aggregate groups of molecules between its own molecules and with molecules of other substances. This greatly increases the surface tension force, which allows water to rise through the soil capillaries and plant vessels.
3. Due to the presence of hydrogen bonds between water molecules, its evaporation requires a large amount of energy, and as a result of its freezing, heat is released. Therefore, the presence of water on our planet in three states of aggregation significantly softens its climate. In addition, many organisms use the evaporation of water at high temperatures to cool their body.
4. Water reaches its greatest density at 4 ° C. Ice has a lower density than water. Therefore, in winter, it is placed on the surface of water bodies and protects the organisms that live in them from hypothermia. Molecules of organic or inorganic substances, which are polar or have charged sites, easily interact with water molecules and, accordingly, easily dissolve in it. Such substances are called hydrophilic. If the molecules of organic or inorganic substances are not polar and do not have charged sites, then they practically do not interact with water molecules and, accordingly, do not dissolve in it. Such substances are called hydrophobic.
Since water in the liquid state still does not have a rigid internal structure, thermal motion molecules leads to constant mixing of molecules aqueous solution. This phenomenon is called diffusion. Due to diffusion, the concentrations of solutes in different parts of the solution are aligned.
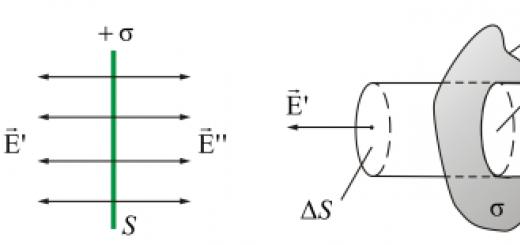
The presence of biological membranes in living organisms leads to the appearance of the phenomenon of osmosis. Owing to the fact that biological membranes is semi-permeable, large particles cannot pass through them organic molecules, but water molecules can pass through. When the concentration of large molecules is different sides membrane is different, water molecules begin to move intensively to the side where the concentration of dissolved substances is higher. As a result, there is an excess of substances on one side of the membrane, which can be observed in the form of osmotic pressure.
Osmotic pressure is very important for living organisms. Thanks to him, turgor (elasticity of plant tissues) arises and cell transport occurs.
Water - the most unique substance, the basis of all living organisms on the planet. It can take various forms and be in three states. What are the main physical and Chemical properties water? It is about them that we will discuss in our article.
Water is...
Water is the most abundant on our planet inorganic compound. The physical and chemical properties of water are determined by the composition of its molecules.
Thus, the structure of a water molecule contains two hydrogen atoms (H) and one oxygen atom (O). Under normal environmental conditions, it is a tasteless, odorless and colorless liquid. Water can also be in other states: in the form of steam or in the form of ice.
More than 70% of our planet is covered by water. Moreover, about 97% falls on the seas and oceans, so most of it is not suitable for human consumption. What are the main chemical properties drinking water- you will learn further.
Water in nature and human life
Water is an essential component of any living organism. In particular, the human body, as is known, consists of more than 70% of water. Moreover, scientists suggest that it was in this environment that life on Earth originated.

Water is contained (in the form of water vapor or droplets) in different layers of the atmosphere. It comes to the earth's surface from the atmosphere in the form of rain or other precipitation (snow, dew, hail, hoarfrost) through condensation processes.
Water is the object of research for a number of scientific disciplines. Among them are hydrology, hydrography, hydrogeology, limnology, glaciology, oceanology and others. All these sciences, one way or another, study the physical and chemical properties of water.
Water is actively used by man in his economic activities, in particular:
- for growing crops;
- in industry (as a solvent);
- in the energy sector (as a coolant);
- to extinguish fires;
- in cooking;
- in pharmacy and so on.
Of course, in order to effectively use this substance in economic activity, the chemical properties of water should be studied in detail.
Varieties of water
As mentioned above, water in nature can be in three states: liquid (actually, water), solid (ice crystals) and gaseous (steam). It can also take on any form.

There are several types of water. So, depending on the content of Ca and Na cations, water can be:
- hard;
- soft.
- fresh;
- mineral;
- brackish.
In esotericism and some religions there is water:
- dead;
- live;
- saint.
In chemistry, there are also such concepts as distilled and deionized water.
The formula of water and its biological significance
Hydrogen oxide is what chemists call this substance. The formula for water is: H 2 O. It means that this compound consists of one oxygen atom and two hydrogen atoms.

The unique chemical properties of water determined its exceptional role for the life of living organisms. It is thanks to water that biological life exists on our planet.
The most unique feature of water is that it perfectly dissolves great amount other substances (both organic and inorganic origin). An important consequence of this feature is that all chemical reactions in living organisms proceed fairly quickly.
In addition, due to the unique properties of water, it is in a liquid state, with an extremely wide temperature range.
Physical properties of water
Thanks to unique hydrogen bonds, water, under standard environmental conditions, is in a liquid state. This explains the extremely high boiling point of water. If the molecules of a substance were not bound by these hydrogen bonds, then the water would boil at +80 degrees, and freeze - as much as -100 degrees.
Water boils at +100 degrees Celsius, and freezes at zero degrees. True, under certain, specific conditions, it can begin to freeze even at positive temperatures. When water freezes, it expands in volume (due to a decrease in density). By the way, this is almost the only substance in nature that has a similar physical property. In addition to water, only bismuth, antimony, germanium and gallium expand upon freezing.
The substance is also characterized by high viscosity, as well as a rather strong surface tension. Water is an excellent solvent for polar substances. You should also know that water conducts electricity through itself very well. This feature is explained by the fact that in water there is almost always a large number of salt ions dissolved in it.
Chemical properties of water (grade 8)
Water molecules have extremely high polarity. Therefore, this substance in reality consists not only of simple molecules type H 2 O, but also from complex aggregates (formula - (H 2 O) n).

Chemically, water is very active, it reacts with many other substances, even at ordinary temperatures. When interacting with oxides of alkali and alkaline earth metals, it forms bases.
Water is also capable of dissolving a wide range of chemical substances- salts, acids, bases, some gases. For this property, it is often called a universal solvent. All substances, depending on whether they dissolve in water or not, are usually divided into two groups:
- hydrophilic (dissolves well in water) - salts, acids, oxygen, carbon dioxide etc.;
- hydrophobic (poorly soluble in water) - fats and oils.
Water also enters into chemical reactions with some metals (for example, sodium), and also takes part in the process of plant photosynthesis.
Finally...
Water is the most abundant inorganic substance on our planet. It is found almost everywhere earth's surface and in its bowels, in the mantle and in rocks, in the high layers of the atmosphere and even in space.
The chemical properties of water are determined by its chemical composition. It belongs to the group of chemical active substances. With many substances, water enters into
Water makes up 70-80% of the mass of living organisms.
The structure of the molecule: the electron density is shifted to oxygen, it has a partial negative charge, on hydrogen it has a partial positive charge, the molecule is a dipole. Hydrogen bonds can form between + and -.
Functions of water
1. Thanks to small dipole molecules, water is the best solvent for polar (hydrophilic) substances. In the dissolved state, substances react very quickly with each other.
2. Transport function: in the dissolved state, substances move around the body.
3. Substances on the surface of which there are no full or partial charges (hydrophobic) cannot interact with water molecules, water pushes them out (fat, gasoline). This is the basis of the structure and work biological membranes.
4. Water has an abnormally high heat capacity(it can absorb a lot of heat and still not get very hot). Due to this, it protects the cell from sudden changes in temperature.
5. Water, like all liquids, incompressible, provides support for cells (turgor) and whole organisms (hydroskeleton).
6. Water itself can participate in chemical reactions as reagent(reactions of hydrolysis, photosynthesis, etc.).
Water is of paramount importance on Earth and throughout the universe.
Water has a very great importance in plant, animal and human life. According to modern ideas, the very origin of life is associated with the sea. In any organism, water is a medium in which chemical processes take place that ensure the vital activity of the organism; in addition, she herself takes part in a number of biochemical reactions. First of all, water can be in three basic states: ice, water and steam. There are over 200 various structures ice that science has discovered.
At the University of Georgia, it was discovered that in any human body, all diseased cells (no matter how sick) are surrounded by water, which is called "unstructured
". It was also found that every healthy cell is surrounded by "structured" water. What does this mean? It's simple, at least in terms of chemistry.
In "unstructured" water, one electron in the outer orbit is simply missing, and in "structured" water there are no missing electrons. Water, when it moves under pressure through the pipes, instead of its natural spiral movement, is forced to move through the pipes in concentric rings. As water moves through pipes, its outer electrons are forced out of orbit, causing the water to become "unstructured". This means that the water from the tap that we drink or in which we bathe in the bathroom gives consequences in the form of diseases. If we take a bath for 20 minutes, we absorb through the skin about 450 grams of water in which we sit. This is equivalent to the fact that we would drink this water. Perhaps humanity is making a mistake much like the one the Romans made when using plates and utensils made of lead.
So, this is the first indication of the difference between "structured" and "unstructured" water.
When this was discovered, many began looking for a way to structure "unstructured" water. For this, magnets, strangely shaped glass vessels, metal nozzles, and the like began to be used all over the world. Our research has shown that artificially structured water, when subjected to energy analysis, does not always look like natural structured water. A magnet, for example, structures water almost instantly, but according to the University of Georgia, it's not safe to drink.
cluster water. About fifteen years ago, completely new water was discovered. It's called "cluster water". Under the microscope, at a magnification of 20 thousand times, the frozen "cluster water" looked like tiny snowflakes. "Cluster water" is found in all newborns, human and other creatures. It is also found in all fruits and vegetables grown without chemical additives. As we get older, the "clustered water" in our bodies at some point combines with proteins. Therefore, we should consume "cluster water" daily to ensure normal water exchange and cell functioning.
Superionized water. Now, however, another new water has become available to the world that could change the world we now know and quite possibly save us from an incredible ecological disaster in future. This water is called "superionized water". Its molecule has three extra electrons in outer orbits and is very stable. If you analyze this new water, you will find nothing but water. But if you take an ordinary lamp and simply dip an electric plug into a glass of this water, the lamp will turn on, and the light from this lamp will be brighter than if you just plugged it into an outlet. It is obvious that this unusual water. It is full of electricity.
No water anywhere!
Water can rightly be called the source of all life on earth. Plants, animals, fish and birds, and of course, the king of nature - man - no one is able to live without water. Some inhabitants of the planet Earth require quite a bit of it, others simply cannot live even an hour without it. A person does not belong to aquatic inhabitants, and only consumes water inside to ensure normal life and uses it for hygiene and pleasure. But it is also directly connected with the water element. 60% of the human body is water. So, adipose tissue takes 20% of the mass of water, bones need 25%, another 70% goes to the liver, skeletal muscles require 75% for themselves, blood needs 80% of water, and the brain needs 85%.
Living organisms live in a constantly changing environment, and for their normal operation, an important component is constancy. internal environment the organism itself. This environment is maintained by blood plasma, tissue fluid, and lymph. And most of them consist of water, proteins and mineral salts. Despite the fact that water and mineral salts are not nutrients and energy sources, metabolic processes are impossible in the absence of water. Water is an excellent solvent. Redox processes and other exchange reactions occur in a liquid medium. Water transports some gases, moving them both in a dissolved state and in the form of salts. Being the content of digestive juices, water helps to remove metabolic products from the body, among which there are toxic substances. Water is involved in thermoregulation.
The importance of water for humans
How many people can live without drinking water? Experts say that no more than 7-10 days. This period is much less than the same experts assign to a person left without food. So water is more important!
Water leaves the human body through the kidneys along with urine. Thus, about 1700 ml is lost. A person loses about 500 ml through the skin. Exhaling through the lungs, a person loses another 300 ml of water.
The ratio between the intake of water and its removal from the body is the water balance. Water balance is very important for the normal functioning of all body systems. In cases where the amount of water drunk is less than a person excretes, there is a risk of developing various disorders. After all, water is part of tissues, as a saline solution is present in the body, it is its structural component and provides a link between water metabolism and mineral substances.
Meaning minerals for the human body
Minerals are an integral part of the skeleton. They are found in the structure of proteins, hormones and enzymes. The total figure of all minerals in the human body is about 4-5% of the mass. Most of the minerals enter the body with food and water. However, the content of minerals in food and water is not always sufficient for the normal functioning of the body. Almost all people season their food table salt, which requires approximately 10-12 grams per day. If there is a chronic lack of minerals in the body, then a person can become seriously ill.
Correct functioning of the central nervous system, heart and other internal organs occurs only in the case of a certain content of mineral ions. Thanks to them, the constancy of osmotic pressure, the reaction of blood and tissue fluid is maintained. Ions of mineral substances take part in the processes of secretion, absorption, excretion and other processes.
Additionally