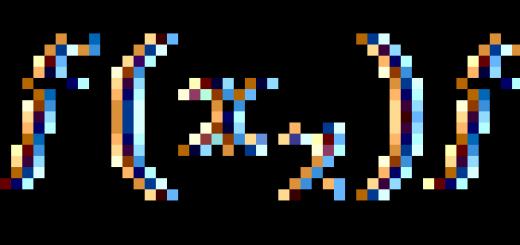1.1 ALKANE (saturated hydrocarbons).
1.2 METHODS FOR OBTAINING ALKANES.
1.3 ALKANE REPRESENTATIVES.
2.1 ALKENES (ethylene hydrocarbons).
2.2 METHODS FOR OBTAINING ALKENES.
2.3 REPRESENTATIVES OF ALKENES.
3.1 ALKYNES (acetylenic hydrocarbons).
3.2 METHODS FOR OBTAINING ALKYNES.
3.3 REPRESENTATIVES OF ALKYNES.
4. APPLICATION OF ALKANE, ALKENES, ALKYNES.
1.1 LIMITED HYDROCARBONS (alkanes).
Saturated hydrocarbons (alkanes) are compounds consisting of carbon and hydrogen atoms, interconnected only by Q-bonds, and not containing cycles. In alkanes, carbon atoms are in the degree of sp3 hybridization.
1.2 Methods for obtaining alkanes.
The main natural source of saturated hydrocarbons is oil, and for the first members of the homologous series, natural gas. However, the isolation of individual compounds from oil or its cracking products is a very laborious and often impossible task, so one has to resort to synthetic methods of production.
1. Alkanes are formed by the action of metallic sodium on monohalogen derivatives - the Wurtz reaction:
H3C-CH2-Br + Br-CH2-CH3 CH3-CH2-CH2-CH3 + 2NaBr
If different halogen derivatives are taken, then a mixture of three different alkanes is formed, since the probability of meeting identical or different molecules in the reaction complex is equal, and their reactivity is close:
3C2H5I + 3CH3CH2CH2IC4H10 + C5H12 + C6H14 + 6NaI
2. Alkanes can be obtained by reducing alkenes or alkynes with hydrogen in the presence of catalysts:
NzS-CH \u003d CH-CHz NzS-CH2-CH2-CH3
3. A wide variety of alkane derivatives can be reduced at high temperature with hydroiodic acid:
H3C H3C
CHBr +2HI CH2 + HBr + I2
H3C H3C
However, in these cases, partial isomerization of the carbon skeleton is sometimes observed - more branched alkanes are formed.
4. Alkanes can be obtained by fusion of salts carboxylic acids with alkali. The resulting alkane contains one carbon atom less than the original carboxylic acid:
O
CH3-C + NaOH CH4 + Na2C03
ONa
1.3 Representatives of alkanes
According to the theory of structure of A. M. Butlerov, the physical properties of substances depend on their composition and structure. Consider, using the example of saturated hydrocarbons, the change physical properties in the homologous series.
The first four members of the homologous series, starting with methane, gaseous substances. From pentane upwards, normal hydrocarbons are liquids. Methane condenses into a liquid only at -162 °C. In subsequent members of the series, the boiling point increases, and when going to the next homologue, it increases by approximately 25 °.
The density of hydrocarbons at the boiling point for the lower members of the series increases first rapidly, and then more slowly: from 0.416 for methane to a value slightly greater than 0.78. The melting point of normal hydrocarbons in the homologous series increases slowly. Starting with the hydrocarbon C16H34, the highest homologues at ordinary temperature are solid substances.
The boiling point of all branched alkanes is lower than that of normal alkanes, and, moreover, the lower the more branched the carbon chain of the molecule. This can be seen, for example, from a comparison of the boiling points of three isomeric pentanes. Conversely, the melting point is highest for the isomers with the most branched carbon chain. Thus, of all isomeric octanes, only the hexa-methyl stage (CH3)3C-C (CH3)3 is solid already at ordinary temperature (mp. 104 ° C). These patterns are explained by the following reasons.
The transformation of a liquid into a gas is prevented by van der Waals forces of interaction between the atoms of individual molecules. Therefore, the more atoms in the molecule, the higher the boiling point of the substance, therefore, in the homologous series, the boiling point should increase evenly. If we compare the interaction forces of n-pentane and neopentane molecules, it is clear that these forces are greater for a molecule with a normal chain of carbon atoms than for branched ones, since in a neopentane molecule the central atom is generally excluded from the interaction.
The main factor affecting the melting point of a substance is the packing density of the molecule in the crystal lattice. The more symmetrical the molecule, the denser its packing in the crystal and the higher the melting point (for n-pentane -132° C, for neopentane -20° C)
2.1 ALKENES (ethylene hydrocarbons, olefins)
Hydrocarbons, in the molecule of which, in addition to simple Q-bonds carbon-carbon and carbon-hydrogen there are carbon-carbon
-bonds are called unlimited. Since the formation of a -bond is formally equivalent to the loss of two hydrogen atoms by a molecule, unsaturated hydrocarbons contain 2n fewer hydrogen atoms than the limiting ones, where n is the number of -bonds
C6H14 C6H12C6H10C6H8C6H6
A series whose members differ from each other by (2H)n is called an isological series. Thus, in the scheme above, the isologues are hexane, hexenes, hexadienes, hexines, hexatrienes, and benzene.
Hydrocarbons containing one bond (i.e., a double bond) are called alkenes (olefins) or, according to the first member of the series, ethylene, ethylene hydrocarbons. The general formula for their homologous series is CnH2n
2.2 Methods for obtaining alkenes
Under the action of alcoholic solutions of caustic alkalis on halogen derivatives: hydrogen halide is split off and a double bond is formed:
H3C-CH2-CH2BrH3C-CH=CH2+NaBr+H2O
Propyl bromide Propylene
If there are tertiary, secondary and primary hydrogen atoms in the α-position to the carbon atom bonded to the halogen, then the tertiary hydrogen atom is predominantly split off, to a lesser extent secondary and even more so primary (Zaitsev's rule):
H3C-C-CI H3C-C + KCL + H2O
H3C CH3 H3C CH3
2,3-Dimethyl-3-chloropentane 2,3-Dimethylpentene-2
This is due to the thermodynamic stability of the resulting alkenes. The more substituents an alkene has on the vinyl carbon atoms, the higher its stability.
2. The effect of water-removing agents on alcohols: a) when alcohols are passed over aluminum oxide at 300-400 ° C.
NzS-CH-CH2.-CHzNzS-CH \u003d CH-CH3
OH Butene-2
sec-butyl alcohol
B) when sulfuric acid acts on alcohols under mild conditions, the reaction proceeds through the intermediate formation of sulfuric acid esters:
H3C-CH-CH3 H3C-CH-CH3 H3C-CH=CH2
OH O-SO3H
isopropyl alcohol
During the dehydration of alcohols under harsh conditions in acidic environments, the same pattern is observed in the elimination of hydrogen atoms different type, as in the elimination of a hydrogen halide.
The first stage of this process is the protonation of alcohol, after which a water molecule is split off and a carbocation is formed:
CH3-CH2-CH-CH3 + H CH3-CH2-CH-CH3 CH3-CH-CH-
OHOH
H H
CH3CH3-CH-CH-CH3CH3-CH=CH-CH3
The resulting carbocation is stabilized by the ejection of a proton from a neighboring position with the formation of a double bond (β-elimination). In this case, the most branched alkene is also formed (thermodynamically more stable). During this process, rearrangements of carbocations associated with the isomerization of the carbon skeleton are often observed:
CH3 CH3
CH3 C-CH – CH3 CH3 C-CH-CH3
CH3 OH CH3
CH3 CH3 CH3 CH3
C-CH C=C
CH3 CH3 CH3 CH3
3. Under the action of Zn or Mg on dihalogen derivatives with two
halogen atoms at neighboring carbon atoms:
H3C – C CH2CIH3C - C - CH2+MgCI2
CH3 CH3
1,2-dichloro-2-metal-isobutylene
propane
4. Hydrogenation of acetylenic hydrocarbons over catalysts with reduced activity (Fe or “poisoned”, i.e. treated with sulfur-containing compounds to reduce catalytic activity, Pt and Pd):
HCC-CH(CH3)2H2C=CH-CH(CH3)2
2.3 Representatives of alkenes.
Like alkaii, the lower homologues of a number of the simplest alkenes under normal conditions are gases, and starting from C5 they are low-boiling liquids (see table).
M.p., T. d4
Formula Name °C Bip, °C
Ch2=CH2 Ethylene -169 -104 0.5660 (at -102°C)
CH3CH \u003d CH3 Propylene -185 -47 0.6090 (at -47 "C)
CH3CH3CH=CH2 CH3-CH=CH-CH3 (cis) Butene-1 -130 -5 0.6696 (at -5°C) 0.6352 (at O°C)
-139 +4
(cis)
CH3-CH=CH-CH3 (trans)-Butep-2 -105 +1 0.6361 (at 0°C)
(trance)
(CH3)3C=CH2 Ieobutylene -140 -7 0.6407 (at 0°C)
All alkenes, like alkanes, are practically insoluble in water and readily soluble in other organic solvents, with the exception of methyl alcohol; they all have a lower density than water.
3.1 ALKYNES (acetylenic hydrocarbons)
Alkynes are hydrocarbons containing, in addition to Q-bonds, two
-bonds (triple bond) on one pair of carbon atoms. The general formula of the homologous series of acetylenic hydrocarbons is СnН2n-2. The formation of one bond is formally equivalent to the loss of two hydrogen atoms.
Various physical methods it has been proved that acetylene C2H2 - I is the simplest representative of the homologous series of alkynes - has a linear molecule in which the length of the carbon-carbon triple bond is 1.20 A, and the length of carbon-hydrogen bonds is 1.06 A.
The CH bonds in acetylene are among the Q bonds formed by overlapping the s orbital of hydrogen with the hybridized sp orbital of carbon; the molecule has one carbon-carbon a-bond (formed by the overlap of two hybridized sp-orbitals of carbon) and two carbon-carbon bonds - the result of the overlap of two mutually perpendicular pairs of "pure" p-orbitals (P and P) of neighboring carbon atoms. Valence angles in acetylene, based on this model, they are 180° and the molecule has a linear conformation, which makes it impossible for cis-trans isomerism with a triple bond.
3.2 Methods for obtaining alkynes.
The most common way to obtain acetylenic hydrocarbons is the action of an alcoholic solution of alkalis on dihalo derivatives of saturated hydrocarbons with a vicinal (a) or geminal (b) arrangement of halogen atoms
a) CH2Br –CH2Br -> CHCH + 2HBr
b) CH3-CH2-CHCl2 -> CH3-CCH+2ISl
CH3-CH2-CCl2-CH3 -> CH3-C C-CH3 + 2HC1
Since vicinal dihalogen derivatives are usually obtained by the addition of halogens to ethylene hydrocarbons, reaction (a) can be considered as a reaction for the conversion of ethylene hydrocarbons into acetylenic ones.
Geminal dihalogen derivatives (both halogen atoms on one carbon atom) are derivatives of ketones or aldehydes and, therefore, using reactions (b), it is possible to make the transition from carbonyl compounds to alkynes. When splitting off hydrogen halides, the already known Zaitsev rule applies that hydrogen is split off from a carbon atom containing a smaller number of hydrogen atoms.
Acetylene can be obtained directly from high-temperature cracking (thermal or electrothermal) of methane or more complex hydrocarbons:
2SN4N-SS-N + ZN2
3.3 Representatives of alkynes.
As with alkanes and alkenes, the lower members of the homologous series of alkynes under normal conditions are gaseous substances. Table data. 22 show that the main physicochemical characteristics of hydrocarbons of the considered classes differ little from each other (see table).
Formula Name Melting point, °С Boiling point, °С D4
HCCHCH3CCHHCC- CH2CH3 CH3CCCH3 Acetylene PropynButyn-1Butyn-2 -82-105-137-33 -84(sub-23) 927 0.6200 (at -84°C) 0.6785 (at -27°C) 0;669b (at -10°C) 0.6880 (at 25°C)
4. APPLICATIONS OF ALKANE, ALKYNE, ALKENES
Alkenes, together with alkanes, acetylene, and aromatic hydrocarbons, are one of the main raw materials for the heavy (large-tonnage) organic synthesis industry.
Ethylene is used in huge quantities for processing into polyethylene and ethyl alcohol, it goes for processing into ethylene glycol and is used in greenhouses to speed up the ripening of fruits.
Propylene is processed into polypropylene, acetone, isopropyl alcohol.
Acetylene plays an extremely important role in industry. Its world production reaches several million tons. A huge amount of acetylene is used for welding metals, when it burns
in oxygen, the temperature reaches 2800 ° C. This is a significantly higher temperature than the combustion of hydrogen in oxygen, not to mention the combustion of methane. The reason for this is the much lower heat capacity of CO2 compared to H2O, which is formed more during the combustion of alkanes than alkynes:
2СзН6 + 7O2 -> 4СО2 + 6Н2О
2C2 H2 + 5O2 -> 4CO2 + 3H2O
The unpleasant smell of acetylene obtained from carbide is due to impurities of PH3 and AsH3, pure acetylene smells like all lower hydrocarbons (gasoline). Acetylene and its mixtures with air are highly explosive; acetylene is stored and transported in cylinders in the form of acetone solutions impregnating porous materials.
OIL AND ITS REFINING
Oil composition. The main natural source of saturated hydrocarbons is oil. The composition of oils varies depending on the field, but all oils are usually separated into the following fractions during simple distillation: gas fraction, gasoline, jet fuel, kerosene, diesel fuel, paraffin, oil tar.
The gas fraction (bp. up to 40? C) contains normal and branched alkanes up to C, mainly propane and butanes. Natural gas from gas fields consists mainly of methane and ethane.
Aviation gasoline (bp. 40-180 ° C) contains hydrocarbons C6 - C10. More than 100 individual compounds have been found in gasoline, including normal and branched alkanes, cycloalkanes and alkylbenzenes (arenes).
Jet fuel (bp 150-280°C).
Tractor kerosene (t, bp 110-300 °C) contains C7-C14 hydrocarbons.
Diesel fuel (bp 200-330 °C), which contains C13 - C18 hydrocarbons, is cracked on a large scale, turning into alkanes (and alkenes) with a lower molecular weight (see below).
Lubricating oils (bp 340-400°C) contain hydrocarbons C18 - C25.
Petroleum paraffin (bp. 320-500 ° C), it contains hydrocarbons C26-C38, from which vaseline is isolated. The residue after distillation is commonly referred to as asphalt or tar.
In addition to hydrocarbons of various classes, oil contains oxygen, sulfur and nitrogen-containing substances; sometimes their total content reaches several percent.
Currently, the most recognized theory is the organic origin of oil as a product of the transformation of plant and animal residues. This is confirmed by the fact that the remains of porphyrins, steroids of plant and animal origin, and the so-called "chemofossils" - the most diverse fragments contained in plankton - were found in oil samples.
Although it is generally recognized that oil is the most valuable natural source of chemical raw materials, the main amount of oil and oil products is still burned in internal combustion engines (gasoline), diesel engines and jet engines (kerosene).
motor fuel. Octane number. Gasolines of various origins behave differently in internal combustion engines.
In an effort to maximize engine power with small dimensions and weight, they try to increase the compression ratio of the combustible mixture in the cylinder. However, in high-speed four-stroke engines operating with forced ignition, this sometimes causes pre-ignition of the mixture - detonation. This reduces the power of the motor and accelerates its wear. This phenomenon is associated with the composition of liquid fuel, since hydrocarbons of different structures behave differently when used as a motor fuel. The worst performance - in paraffins of normal structure.
Normal heptane was adopted as the standard for a combustible substance with a high detonation ability. The more branched the carbon chain of the paraffinic hydrocarbon, the better its combustion in the cylinder proceeds and the greater the degree of compression of the combustible mixture can be achieved. 2, 2, 4-trimethylpentane (commonly referred to as isooctane) with good anti-knock properties has been adopted as a motor fuel standard. Composing mixtures of this octane with n-heptap in various proportions, their behavior in the engine is compared with the behavior of the tested gasoline. If a mixture containing 70% isooctane behaves in the same way as the gasoline under study, then the latter is said to have an octane number of 70 (the octane number of isooctane is taken as 100; the octane number of n-heptane is taken to be zero).
One of the ways to increase the knock resistance of fuels for engines with spark ignition is the use of antiknock agents.
Antiknock agents are substances that are added to gasolines (not more than 0.5%) to improve antidetonation properties. A sufficiently effective antiknock agent is tetraethyl lead (TES) Pb (C2H5)4
However, gasoline from thermal power plants and its combustion products are very toxic. At present, new antiknock agents based on organic manganese compounds of the type cyclopentadieneiclpsntacarbonyl manganese C5H5Mn (CO)5 have been found: they are less toxic and have better antiknock properties. The addition of these anti-knock agents to good grades of gasoline produces fuel with an octane rating of up to 135.
For rocket and diesel engines, on the contrary, fuels with a normal chain of carbon atoms, which have the lowest ignition temperature, are most valuable. This characteristic is taken
evaluate in cetane numbers. The cetane number 100 has the hydrocarbon n-Sc, Hd4, and the cetane number 0 has 1-methylnaphthalene.
Synthesis of hydrocarbons from CO+H2. By passing a mixture of carbon monoxide (II) and hydrogen over finely crushed nickel at 250 ° C, methane can be obtained:
CO+3H2CH4+H2O
If this reaction is carried out at a pressure of 100-200 atm and a temperature of up to 400 ° C, a mixture is obtained, consisting mainly of oxygen-containing products, among which alcohols predominate; this mixture was called schshpol.
When using iron-cobalt catalysts and a temperature of 200 ° C, a mixture of alkanes is formed - syntin.
nCO + (2n + 1) H2 CnH2n + 2 + H2O
Sintin and synthol are products of large-scale organic synthesis and are widely used as raw materials for many chemical industries.
Clathrates. Sintin and gasoline fractions of oil consist of mixtures of hydrocarbons with a normal structure and branched chains. Was recently found effective method separation of organic compounds with normal chains and branched ones, obtained in general case the name of the clathrate separation method. Urea was used to separate hydrocarbons. Urea crystals are built in such a way that there are narrow hexagonal channels inside the crystals. The diameter of these channels is such that only normal hydrocarbons can pass through and be retained by adsorption forces. Therefore, when a mixture of organic compounds is treated with urea (or some other compounds), substances with a normal chain of carbon atoms crystallize together with it in the form of complexes. This method has, of course, a very big future - when it will be found more effective clathrate formers.
PRODUCTION OF BUTADIENE-1,3 (DIVINYL)
Butadiene-1,3 CH 2 = CH-CH-CH 2 is the main monomer for the production of synthetic rubbers.
The synthesis of butadiene-1,3 from ethanol, developed by S. V. Lebedev, was the first industrial method for producing a monomer, on the basis of which in 1932 a plant for the production of synthetic rubber was launched for the first time in the world.
The overall reaction equation can be written as
2C 2 H 5 OH ® C 4 H 6 + H 2 + 2H 2 O, ΔH = 85 kJ
It can be seen from the equation that the overall reaction is a combination of condensation, dehydrogenation and dehydration. These requirements are met by the bifunctional oxide catalyst proposed by Lebedev, which contains dehydrogenating and dehydrating components. However, now the method has lost its practical value. The principal disadvantage of the method is its low selectivity (even the theoretical yield of divinyl from 100% ethanol is 58.7%).
Currently, the main methods for the synthesis of divinyl are dehydrogenation n- butane isolated from natural gas and complex processing of butane-butylene pyrolysis fractions of petroleum products, including butadiene extraction, isobutylene isolation and dehydrogenation n-butylenes to butadiene.
In the dehydrogenation of butane, thermodynamic limitations play a significant role, as a result of which it is almost impossible to obtain butadiene-1,3 in one stage with a technically acceptable yield under normal conditions, and only with the help of special methods (using a vacuum, oxidative dehydrogenation) can the yield be raised to the required level .
Most industrial installations for the production of divinyl from butane operate in a two-stage scheme. The first stage of dehydrogenation of butane is to convert it to butylene, and the second is the process of obtaining divinyl from butylene.
The dehydrogenation of butane to butylene on a chromium oxide promoted catalyst supported on alumina proceeds according to the reaction
C 4 H 10 ® C 4 H 8 + H 2, ΔH = 131 kJ
Composition ...... Al 2 O 3 Fe 2 O 3 Cr 2 O 3 SiO 2 KNO 3 CaO H 2 O
Mass fraction, % 66,10 1,72 15,8 7,9 4,93 0,14 3,34
In the process of butane dehydrogenation, the catalyst is covered with carbon deposits and changes its chemical composition. In this case, the activity of the catalyst sharply decreases. For the purpose of reactivation, the catalyst is continuously withdrawn from the reactor and calcined in a stream of air in a fluidized bed regenerator. In this case, carbonaceous compounds burn out, and lower chromium oxides are oxidized to Cr 2 Oz. Technology system butane dehydrogenation unit is shown in fig. one.
Butane in liquid form enters the dryer 1 filled with an adsorbent (A1 2 O 3, zeolites) and then into the evaporator 2. The resulting vapors are heated in a tube furnace 3 up to a temperature of 780-820 K and enter under the distribution grid of the reactor 4 for dehydration. The amount of heat necessary for the reaction to proceed is supplied with the flow of heated regenerated catalyst from the regenerator 5. The temperature in the regenerator is 890-920 K. The regenerated catalyst is fed to the upper distribution grate and, therefore, the catalyst and reaction gases move countercurrently. In the upper part there is a coil for quenching the reaction gases. Due to this, the temperature of gases quickly decreases to 720-750 K and their further decomposition is prevented.
| |
The catalyst is transported to the regenerator by an air stream, and to the reactor by the initial hydrocarbon vapors or nitrogen. The contact gas from the reactor is sent to the waste heat boiler 6 to obtain secondary steam, and then to trap the catalyst dust and further cooling - into the scrubber 7, irrigated with water. Flue gases from the regenerator are freed from catalyst dust in an electrostatic precipitator 8, then pass through the scrubber and are released into the atmosphere.
To make up for losses and maintain activity, fresh catalyst is added daily to the catalyst circulating in the system. The purified contact gas enters the turbocharger 9, the discharge pressure of which is about 0.5 MPa, and then into the condensing system 10, where water and boiling propane are used in succession as a refrigerant. The non-condensed product is sent to the absorber 11 . Absorption is carried out by a mixture of hydrocarbons C 6 -C 12 . Dissolved butylene is distilled off in a desorber 12 and mixtures with liquefied product from the condenser 10 enters the system of distillation columns 13 and 14. In columns, low- and high-boiling impurities are distilled off from the dehydrogenation product (the latter are added to the circulating absorbent to compensate for losses.). Butane dehydrogenation products are sent to the extractive distillation unit 15 to isolate the butylene fraction.
The dehydrogenation of butylene to divinyl proceeds on
calcium chromium phosphate catalyst by the reaction
C 4 H 8 ® C 4 H 8 + H 2, ΔH=119kJ.
The technological scheme of butylene dehydrogenation is shown in Fig.2.
| |
 The original butylene fraction and water vapor are superheated in tube furnaces 1
and 2
up to 770 and 990 K, respectively, mixed directly before the reactor in an injection mixer 3
and sent to the reactor block 4.
The gas-steam mixture at the outlet of the reactor is "quenched" with water condensate, immediately cooling to 810 K. Each reactor is equipped with a waste heat boiler 5, after passing through which the contact gas is additionally cooled and cleaned in a system of two scrubbers 6
and 7, the first of which is irrigated with diesel fuel and the second with water. Water vapor is completely condensed in scrubbers. After leaving the scrubber 7, the gas is compressed in the compressor 8
and condenses in the condensing system 9.
Uncondensed hydrocarbons are additionally recovered in the absorber-desorber unit 10
and 11
. The absorbent are C 6 -C 12 hydrocarbons formed as by-products. The total liquefied flow is sent to the columns 12
and 13
for preliminary separation of low- and high-boiling impurities and further to the extractive distillation unit 14.
The conversion of butylene averages 40-45% with a selectivity for divinyl of about 85%.
The original butylene fraction and water vapor are superheated in tube furnaces 1
and 2
up to 770 and 990 K, respectively, mixed directly before the reactor in an injection mixer 3
and sent to the reactor block 4.
The gas-steam mixture at the outlet of the reactor is "quenched" with water condensate, immediately cooling to 810 K. Each reactor is equipped with a waste heat boiler 5, after passing through which the contact gas is additionally cooled and cleaned in a system of two scrubbers 6
and 7, the first of which is irrigated with diesel fuel and the second with water. Water vapor is completely condensed in scrubbers. After leaving the scrubber 7, the gas is compressed in the compressor 8
and condenses in the condensing system 9.
Uncondensed hydrocarbons are additionally recovered in the absorber-desorber unit 10
and 11
. The absorbent are C 6 -C 12 hydrocarbons formed as by-products. The total liquefied flow is sent to the columns 12
and 13
for preliminary separation of low- and high-boiling impurities and further to the extractive distillation unit 14.
The conversion of butylene averages 40-45% with a selectivity for divinyl of about 85%. CHLORINATION OF PARAFFINS AND THEIR HALIDEDERIVATIVES
In industry, thermal chlorination is carried out in the gas phase at a temperature necessary for the activation of chlorine molecules, giving rise to a radical chain reaction: C1 2 ® C1× + C1×
RH + C1× ® R + HCl
R + Cl 2 ® RCl + C1×, etc.
The reaction of substitution of hydrogen atoms for chlorine atoms leads to
to the formation of a mixture of mono-, di- and polychlorine products and the release of hydrogen chloride.
The predominant formation of one or another product is determined by the reaction conditions; temperature regime and molecular ratio of hydrocarbon and chlorine (Fig. 3).

Chlorination of methane is carried out in a chlorinator (Fig. 4), which is a steel cylindrical body, lined from the inside with fireclay bricks 2, in the upper part of which there is a nozzle made of porcelain rings 3, contributing to a uniform reaction. Half the height of the interior of the chlorinator is occupied by an open ceramic vertical cylinder 4 with holes at the bottom, into which a ceramic pipe is lowered with a narrowed ring, supplying raw materials. The process begins by preheating the inside of the chlorinator (to initiate the reaction). Heating is carried out by burning part of the methane mixed with air, followed by the replacement of air with chlorine. Subsequently, the reaction proceeds autothermally. Chlorination products are discharged from the upper part of the apparatus, then hydrogen chloride is captured from the gas mixture in acid absorbers (hydrochloric acid is obtained), the gas mixture is neutralized with alkali, freeze-dried, compressed and liquefied by deep cooling. From a liquid mixture containing 28-32% methyl chloride, 50-53% methylene chloride, 12-14% chloroform and 3-5% carbon tetrachloride, individual products are isolated by rectification.
All chlorine-substituted methane find wide application. So, methyl chloride CH 3 C1 is used as a solvent in the production of butyl rubber, as a methylating agent in organic synthesis, to obtain methylchlorosilanes, which serve as feedstock in the production of organosilicon polymers - silicones. Methylene chloride CH 2 C1 2 is a valuable industrial solvent for cellulose acetate, fats, oils, paraffin, rubbers; it is non-flammable and does not form explosive mixtures with air.
Benzene Chlorination
By chlorination of benzene, monochlorobenzene or other chlorine derivatives are obtained, depending on the chlorination conditions. Thus, at 310–330 K and a molar ratio of benzene and chlorine of 1:0.6, monochlorobenzene is formed on an iron catalyst; with a lower ratio and catalyst A1C1 3, mainly o-dichlorobenzene is obtained (used in the synthesis of dyes and pest control agents); at the same temperature under ultraviolet irradiation, hexachlorocyclohexane is obtained. On fig. Figure 5 shows a scheme for the production of chlorobenzene with the removal of the heat of the exothermic reaction due to the evaporation of excess benzene.
 Chlorination is carried out in a steel cylindrical apparatus,
Chlorination is carried out in a steel cylindrical apparatus,
| |
THE PROBLEM OF THE USE OF HYDROGEN CHLORINE PRODUCED IN THE PROCESSES OF HYDROCARBONS CHLORINATION
Hydrogen chloride is a waste product of the chlorination of paraffinic and aromatic hydrocarbons in oil refining, which is widely used in industrial organic synthesis. Recycling is an urgent task associated with reducing the cost of chlorination products, improving sanitary conditions, and combating metal corrosion.
Part of the hydrogen chloride is used to produce of hydrochloric acid by countercurrent absorption of HC1 into water. However, local demand for hydrochloric acid is usually much less than the capacity to produce it from hydrogen chloride. The transport of hydrochloric acid over long distances is difficult due to its high corrosivity.
A promising way to utilize HC1 is the oxidative chlorination method. This method is used in modern industry to synthesize vinyl chloride from ethylene: in an oxidative chlorination reactor, ethylene is converted into 1,2-dichloroethane, by catalytic decomposition of which vinyl chloride is obtained; the resulting HC1 is again sent to the reactor:
2CH 2 \u003d CH 2 + 4HC1 + O 2 ® 2CH 2 C1-CH 2 C1 + 2H 2 O, ΔH \u003d -238 kJ / mol CH 2 C1-CH 2 C1 ® CH 2 \u003d CHCI + HC1.
The process of oxidative chlorination takes place at 530-570 K in the presence of a catalyst (copper chloride on an inert carrier); pyrolysis of dichloroethane is carried out at 770 K on a porous catalyst (pumice stone).
| |

On fig. 6 shows a simplified scheme for the synthesis of vinyl chloride from ethylene. In the mixer 1 ethylene, recycle gas and hydrogen chloride are mixed with oxygen and fed into the reactor 2 with fluidized catalyst; the vapors of the formed dichloroethane and unreacted ethylene, oxygen and HC1 are cooled in a direct mixing refrigerator 3 a mixture of water and dichloroethane coming from the refrigerator 4. Then the gas-vapor mixture passes through a hot alkaline scrubber 5, in which it is purified from HCl and CO 2 , cooled in a refrigerator and, passing through a gas separator 6, separated from gases - a mixture of ethylene and oxygen, which are returned to the reactor (recycle gas). Dichloroethane in the separator 7 is separated from the water, enters the drying column 8, where, with the help of azeotropic distillation, it is finally dehydrated and fed into a distillation column 9; dichloroethane is collected in a collection 10. The subsequent pyrolysis of dichloroethane to obtain vinyl chloride occurs in a tube furnace 11 ; the reaction mixture from the furnace enters the direct mixing refrigerator, is cooled by circulating cooled dichloroethane and, after passing through the refrigerator 4, enters the distillation column 12, where HC1 is separated, which is returned to the oxidative chlorination reactor, and vinyl chloride and unconverted dichloroethane are separated in a distillation column 13; dichloroethane is returned to the column 9, and vinyl chloride goes to the polymerization.
Of considerable interest for recycling is the combination of enterprises based on refinery gases, in particular the joint processing of ethylene and acetylene and vinyl chloride; Hydrogen chloride, formed during the production of vinyl chloride from ethylene, is used to hydrochlorinate acetylene:
CH 2 \u003d CH 2 + C1 2 ® CH 2 C1-CH 2 C1 (pyrolysis)® CH 2 \u003d CHC1 + HCI
CHºCH + HC1 ® CH 2 = CHC1
An economical way to utilize hydrogen chloride is to combine methane chlorination with oxidative chlorination in order to obtain chlorine-substituted methane:
CH 4 + 4C1 2 ® CCI 4 + 4HCI
CH 4 + 4HC1 + O 2 ® SS! 4 + 2H 2 O
In this process, in addition to carbon tetrachloride, methylene chloride and chloroform are obtained. Carbon tetrachloride is used as a solvent, in agriculture (fumigant), for extinguishing fires, etc. Chloroform is a valuable intermediate product in the synthesis of phenols, fluoroplastics, etc.
Oxidative chlorination also produces chlorobenzene from a gas-vapor mixture of benzene, hydrogen chloride and air (oxygen) at 500 K on a mixed catalyst (A1 2 O 3 -CuC1 2 - FeCl 3):
C 6 H 6 + HC1 + 1/ 2 O 2 ® C 6 H 5 C1 + H 2 O
Hydrogen chloride can be utilized by its electrochemical oxidation to chlorine.
A chromium cesium catalyst and a method for using it for the oxidation of hydrogen chloride to chlorine, i.e., the regeneration of chlorine from the exhaust gases of the chlorination of organic compounds, is proposed.
ACETYLENE PRODUCTION AND ITS PROCESSING
The production of acetylene by decomposition of calcium carbide is carried out in acetylene generators by wet and dry methods according to the reaction equation:
CaC 2 + 2H 2 O ® C 2 H 2 + Ca (OH) 2 ΔH = -127 kJ.
In the wet method, in generators operating on the “carbide to water” principle, crushed calcium carbide is evenly fed into the generator containing a large amount of water, by heating which the heat released during the process is removed. The equipment used according to this scheme, and especially communications for removing the resulting sludge and water circulation, are very cumbersome. In addition, transportation and use of liquid lime milk containing up to 70% water cause great difficulties.
Efficient industrial methods for producing acetylene from hydrocarbons have also been developed. Acetylene is formed from paraffins by the following reversible endothermic reactions:
2CH 4 D C 2 H 2 + H 2 ΔH = 376 kJ
C 2 H 6 D C 2 H 2 + 2H 2 ΔH = 311 kJ
C 3 H 8 D C 2 H 2 + CH 4 + H 2 ΔH = 255 kJ
CH 4 D C + 2H 2 ΔH = 88 kJ
Reaction (d) is a side reaction.
The equilibrium of reactions with increasing temperature shifts towards the formation of acetylene. A high degree of equilibrium conversion for methane is achieved at T>1670 K, for ethane - 1170 K. But at temperatures >1680 K, acetylene and hydrocarbons become unstable and decompose into soot and carbon.
The reaction of the conversion of methane into acetylene at temperatures of 1670-1770 K accepted in production is faster than the reaction of decomposition of acetylene into elements, therefore the reaction products are quickly cooled, which makes it possible to prevent the decomposition of acetylene, for the same purpose, high gas volumetric velocities are used, at which the raw material must be in reaction zone only thousandths of a second.
According to the method of heat supply for the implementation of the exothermic reaction of the formation of acetylene, the following methods of carrying out the process are distinguished: 1) electrocracking of gaseous hydrocarbons or liquid products; 2) homogeneous pyrolysis; 3) thermal-oxidative pyrolysis.
electrocracking It is carried out using a voltaic arc in DC electric arc furnaces.
Homogeneous pyrolysis consists in the decomposition of raw materials in a stream of hot flue gases at a temperature of about 2200 K.
In thermal oxidative pyrolysis the necessary heat is obtained by burning part of the methane.
The main disadvantages of the carbide method for producing acetylene are the high power consumption in the production of calcium carbide and a significant amount of consumed raw materials (limestone and coke) processed in several stages. At the same time, with the carbide method, concentrated acetylene is obtained, the purification of which from small impurities does not cause difficulties.
The methods of thermal decomposition of hydrocarbons use a smaller amount of feedstock, which is converted to acetylene in one stage, but the acetylene is diluted and requires a complex system its purification and concentration. It should be noted that the carbide method provides about 70% of the world production of acetylene.
There are the following main methods for the primary processing of acetylene.
Hydration:
a) with the production of acetaldehyde and acetic acid (catalyst (HgSO 4):
b) with the production of acetone (ZnO catalyst on activated carbon)
2CH \u003d CH + 3H 2 O ® CH 3 COCH 3 + CO 2 + 2H 2
Polymerization into linear and cyclic substances to obtain synthetic rubber monomers and fibers.
Chlorination to obtain solvents and monomers.
Vinylation with acetylene various substances to obtain monomers:
ROH ® ROCH=CH 2
RCOOH ® RCOOCH=CH 2
Ministry of Education R.F.
Kursk state agricultural
academy. Prof. I. I. Ivanova
ABSTRACT ON
organic chemistry
OBTAINING ALKANES, ALKENES, ALKYNS.
KEY REPRESENTATIVES.
APPLICATION IN INDUSTRY.
Completed:
KURSK-2001
Plan.
1.1 ALKANE (saturated hydrocarbons).
1.2 METHODS FOR OBTAINING ALKANES.
1.3 ALKANE REPRESENTATIVES.
2.1 ALKENES (ethylene hydrocarbons).
2.2 METHODS FOR OBTAINING ALKENES.
2.3 REPRESENTATIVES OF ALKENES.
3.1 ALKYNES (acetylenic hydrocarbons).
3.2 METHODS FOR OBTAINING ALKYNES.
3.3 REPRESENTATIVES OF ALKYNES.
4. APPLICATION OF ALKANE, ALKENES, ALKYNES.
1.1 LIMITED HYDROCARBONS (alkanes).
Saturated hydrocarbons (alkanes) are compounds consisting of atoms
carbon and hydrogen, interconnected only by Q-bonds, and not containing
cycles. In alkanes, carbon atoms are in the degree of hybridization sp3.
1.2 Methods for obtaining alkanes.
The main natural source of saturated hydrocarbons is oil, and for
the first members of the homologous series are natural gas. However, selection
individual compounds from oil or its cracking products are very
time-consuming, and often impossible task, so you have to resort to
synthetic methods of obtaining.
1. Alkanes are formed under the action of metallic sodium on
monohalogen derivatives - wurtz reaction:
H3C-CH2-Br + Br-CH2-CH3 CH3-CH2-CH2-CH3 + 2NaBr
If different halogen derivatives are taken, then a mixture of three different
alkanes, since the probability of meeting molecules in the reaction complex
identical or different is equal, and their reactivity is close:
3C2H5I + 3CH3CH2CH2IC4H10 + C5H12 + C6H14 + 6NaI
2. Alkanes can be obtained in the reduction of alkenes or alkynes
hydrogen in the presence of catalysts:
NzS-CH \u003d CH-CHz NzS-CH2-CH2-CH3
3. A wide variety of alkane derivatives can be restored at
high temperature hydroiodic acid:
CHBr +2HI CH2 + HBr + I2
However, in these cases, partial isomerization of the carbon
skeleton - more branched alkanes are formed.
4. Alkanes can be obtained when fusing salts of carboxylic acids with
alkali. The resulting alkane contains one less carbon atom,
than the original carboxylic acid:
CH3-C + NaOH CH4 + Na2C03
1.3 Representatives of alkanes
According to the theory of structure of A. M. Butlerov, the physical properties of substances depend
on their composition and structure. Consider the example of saturated hydrocarbons
change in physical properties in the homologous series.
The first four members of the homologous series, starting with methane, are gaseous
substances. From pentane and up, normal hydrocarbons are
a liquid. Methane condenses into a liquid only at -162 °C. Subsequent
members of the series, the boiling point increases, and upon transition to the next
to the homologue, it increases by approximately 25°.
The density of hydrocarbons at the boiling point for the lower members of the series
increases rapidly at first, and then more slowly: from 0.416 for methane to
values slightly greater than 0.78. The melting point of normal
hydrocarbons in the homologous series increases slowly. Beginning with
hydrocarbon С16Н34, higher homologues at ordinary temperature - substances
The boiling point of all branched alkanes is lower than that of normal
alkanes, and, moreover, the lower, the more branched the carbon chain of the molecule.
This can be seen, for example, from a comparison of the boiling points of three isomeric pentanes.
Conversely, the melting point is highest for isomers with
the most branched carbon chain. So, from all isomeric octane
only the hexa-methyl stage (CH3)3C-C (CH3)3 is a solid even at
normal temperature (mp. 104 ° C). These patterns are explained
the following reasons.
The transformation of a liquid into a gas is prevented by van der Waals interaction forces
between the atoms of individual molecules. Therefore, the more atoms in a molecule, the higher
the boiling point of a substance, therefore, in the homologous series, the temperature
boiling should increase evenly. If we compare the forces of interaction of molecules
n-pentane and neopentane, it is clear that these forces are greater for a molecule with
a normal chain of carbon atoms than for branched ones, since in a molecule
neopentane, the central atom is generally excluded from the interaction.
Density is the main factor affecting the melting point of a substance.
packing of a molecule in a crystal lattice. The more symmetrical the molecule, the
the denser its packing in the crystal and the higher the melting point (at n
Pentane -132° C, neopentane -20° C)
2.1 ALKENES (ethylene hydrocarbons, olefins)
Hydrocarbons, in the molecule of which, in addition to simple Q-bonds, carbon - carbon and
carbon - hydrogen there are carbon-carbon
The connections are called
unlimited. Since education is
bond is formally equivalent to the loss of two hydrogen atoms by the molecule, then
unsaturated hydrocarbons contain 2p fewer hydrogen atoms than
limit, where n is a number
C6H14 C6H12C6H10C6H8C6H6
A series whose members differ from each other by (2Н)n is called
isological side. So, in the above scheme, the isologists are
hexane, hexenes, hexadienes, hexines, hexatrienes and benzene.
hydrocarbons containing one
A bond (i.e. a double bond) is called alkenes (olefins) or, by
the first member of the series - ethylene, ethylene hydrocarbons. General formula
their homologous series - CnH2n
2.2 Methods for obtaining alkenes
Under the action of alcoholic solutions of caustic alkalis on halogen derivatives:
hydrogen halide is cleaved off and a double bond is formed:
H3C-CH2-CH2BrH3C-CH=CH2+NaBr+H2O
Propyl bromide Propylene
If in the α-position to the carbon atom bonded to the halogen is
tertiary, secondary and primary hydrogen atoms, then it is predominantly split off
tertiary hydrogen atom, to a lesser extent secondary and even more so primary
(Zaitsev's rule):
H3C-C-CI H3C-C + KCL + H2O
2,3-Dimethyl-3-chloropentane 2,3-Dimethylpentene-2
This is due to the thermodynamic stability of the resulting alkenes. How
the more substituents an alkene has on vinyl carbon atoms, the higher its
sustainability.
2. Action on alcohols of water-removing agents: a) when passing
alcohols over aluminum oxide at 300-400°C.
NzS-CH-CH2.-CHzNzS-CH \u003d CH-CH3
Deut-butyl alcohol
b) when sulfuric acid acts on alcohols under mild conditions, the reaction proceeds
through the intermediate formation of sulfuric acid esters:
H3C-CH-CH3 H3C-CH-CH3 H3C-CH=CH2
isopropyl alcohol
During the dehydration of alcohols under severe conditions in acidic media, the same
regularity in the splitting off of hydrogen atoms of various types, as in
elimination of hydrogen halide.
The first stage of this process is the protonation of alcohol, after which
a water molecule is split off and a carbocation is formed:
CH3-CH2-CH-CH3 + H CH3-CH2-CH-CH3 CH3-CH-CH-
CH3CH3-CH-CH-CH3CH3-CH=CH-CH3
The resulting carbocation is stabilized by the ejection of a proton from the neighboring
positions with the formation of a double bond (β-elimination). In that
case, the most branched alkene is also formed (thermodynamically more
stable). During this process, rearrangements of carbocations are often observed.
associated with the isomerization of the carbon skeleton:
CH3 C-CH – CH3 CH3 C-CH-CH3
CH3 CH3 CH3 CH3
3. Under the action of Zn or Mg on dihalogen derivatives with two
halogen atoms at neighboring carbon atoms:
H3C – C CH2CIH3C - C - CH2+MgCI2
1,2-dichloro-2-metal-isobutylene
4. Hydrogenation of acetylenic hydrocarbons over catalysts with
reduced activity (Fe or "poisoned", i.e. processed
HCC-CH(CH3)2H2C=CH-CH(CH3)2
2.3 Representatives of alkenes.
Like alkaii, the lower homologues of a number of the simplest alkenes under normal conditions are
gases, and starting from C5 - low-boiling liquids (see table).
| m.p., | T. | d4 | ||
| Formula | Name | °C | Kip., °С | |
| Ch2=CH2 | Ethylene | -169 | -104 | 0.5660 (at -102°C) |
| CH3CH=CH3 | Propylene | -185 | -47 | 0.6090 (at -47 "C) |
| CH3CH3CH=CH2 CH3-CH=CH-CH3 | (cis)Butene-1 | -130 | -5 | 0.6696 (at -5°C) 0.6352 (at O°C) |
| -139 | +4 | |||
(cis) | ||||
| CH3-CH=CH-CH3 | (trans)-Butep-2 | -105 | +1 | 0.6361 (at 0°C) |
(trance) | ||||
| (CH3)3C=CH2 | iobutylene | -140 | -7 | 0.6407 (at 0°C) |
All alkenes, like alkanes, are practically insoluble in water and readily soluble.
in other organic solvents, with the exception of methyl alcohol; all
they have a lower density than water.
3.1 ALKYNES (acetylenic hydrocarbons)
Alkynes are hydrocarbons containing, in addition to Q-bonds, two
Ties (triple
bond) on one pair of carbon atoms. General formula of the homologous series
acetylenic hydrocarbons СnН2n-2 formation of one
A bond is formally equivalent to the loss of two hydrogen atoms.
It has been proven by various physical methods that acetylene C2H2 - I is the simplest
representative of the homologous series of alkynes - has a linear molecule,
in which the length of the carbon-carbon triple bond is 1.20 A, and the bond length
carbon-hydrogen 1.06 A.
The C-H bonds in acetylene are among the Q-bonds formed by
overlapping of the hydrogen s-orbital with the hybridized sp- orbital
carbon; there is one carbon-carbon a-bond in the molecule (formed by
overlap of two hybridized sp-orbi- carbon hoists) and two
carbon-carbon
Connections are the result of overlapping of two mutually perpendicular pairs of "pure"
p-orbitals (R
neighboring carbon atoms. Bond angles in acetylene based on this model
are equal to 180° and the molecule has a linear conformation, which makes it impossible
cis-trans isomerism at the triple bond.
3.2 Methods for obtaining alkynes.
The most common way to obtain acetylenic hydrocarbons is
the effect of an alcoholic solution of alkalis on dihalogen derivatives of limiting
hydrocarbons with vicinal (a) or geminal (b) arrangement of atoms
halogen
a) CH2Br -CH2Br -> SNSN + 2НВг
b) CH3-CH2-CHCl2 -> СНЗ-ССН + 2ISl
CH3-CH2-CCl2-CH3 -> CH3-C C-CH3 + 2HC1
Since vicinal dihalogen derivatives are usually obtained by adding
halogens to ethylene hydrocarbons, then reaction (a) can be considered as
the reaction of converting ethylene hydrocarbons into acetylenic ones.
Geminal dihalogen derivatives (both halogens on one carbon atom)
are derivatives of ketones or aldehydes and, therefore, with the help of
reactions (b) it is possible to carry out the transition from carbonyl compounds to alkynes.
When splitting off hydrogen halides, the already known Zaitsev rule applies, which
hydrogen is split off from a carbon atom containing a smaller amount
hydrogen atoms.
Acetylene can be obtained directly from high-temperature cracking
(thermal or electrothermal) methane or more, complex
hydrocarbons:
2SN4N-SS-N + ZN2
3.3 Representatives of alkynes.
As with alkanes and alkenes, the lower members of the homologous series of alkynes in ordinary
gaseous conditions. Table data. 22 show that the main
physical and chemical characteristics of hydrocarbons of the considered classes are few
differ from each other (see table).
| Formula | Name | T. pl., ° С | T boil., °С | D4 |
HCC-CH2CH3 CH3CCCH3 | Acetylene Propyne | (air,-23) 9 | 0.6200 (at -84°C) 0.6785 (at -27°C) 0;669b (at -10°C) 0.6880 (at 25°C) | |
4. APPLICATIONS OF ALKANE, ALKYNE, ALKENES
Alkenes along with alkanes, acetylene and aromatic hydrocarbons
are one of the main raw material sources of heavy industry
(multi-tonnage) organic synthesis.
Ethylene is used in large quantities for processing into polyethylene and
ethyl alcohol, it goes for processing into ethylene glycol and is used in
greenhouses to accelerate the ripening of fruits.
Propylene is processed into polypropylene, acetone, isopropyl alcohol.
Acetylene plays an extremely important role in industry. His world
production reaches several million tons. Huge amount
acetylene is used to weld metals, when it burns
in oxygen, the temperature reaches 2800 ° C. This is a much higher
temperature than the combustion of hydrogen in oxygen, not to mention the combustion
methane. The reason for this is the much lower heat capacity of CO2 compared to
H2O, which is formed more during the combustion of alkanes than alkynes:
2СзН6 + 7O2 -> 4CO2 + 6H2O
2C2 H2 + 5O2 -> 4CO2 + ZH2O
The unpleasant odor of carbide-derived acetylene is due to PH3 impurities.
and AsH3, pure acetylene smells like all lower hydrocarbons (gasoline).
Acetylene and its mixtures with air are highly explosive; acetylene is stored and
transported in cylinders in the form of acetone solutions impregnating
porous materials.
OIL AND ITS REFINING
Oil composition. The main natural source of saturated hydrocarbons
is oil. The composition of oils varies depending on the field,
however, all oils in simple distillation are usually separated into the following fractions:
gas fraction, gasoline, jet fuel, kerosene, diesel fuel,
paraffin, oil tar.
gas fraction(bp. up to 40◦C) contains normal and
branched alkanes up to C, mainly propane and butanes. natural gas from
gas fields consists mainly of methane and ethane.
Aviation gasoline(bp. 40-180 ° C) contains hydrocarbons
C6 - C10 More than 100 individual compounds have been found in gasoline,
which include straight and branched alkanes, cycloalkanes and
alkylbenzenes (arenes).
jet fuel(bp 150-280°C).
Tractor kerosene(t, bp 110-300 °C) contains C7-C14 hydrocarbons.
Diesel fuel(bp. 200-330 ° C), which includes
hydrocarbons C13 - C18, are cracked on a large scale, turning
into alkanes (and alkenes) with lower molecular weights (see below).
Lubricating oils(bp 340-400°C) contain hydrocarbons C18 - C25.
Petroleum paraffin(bp. 320-500 ° C), it contains hydrocarbons
C26-C38, from which vaseline is isolated. The residue after distillation is usually called
asphalt or tar.
In addition to hydrocarbons of various classes, oil contains oxygen,
sulfur and nitrogen-containing substances; sometimes their total content reaches
up to several percent.
Currently, the most recognized is the theory of organic
the origin of oil as a product of the transformation of plants and animals
leftovers. This is confirmed by the fact that residues were found in the oil samples.
porphyrins, steroids of plant and animal origin and the so-called
"chemofossils" - the most diverse fragments contained in plankton.
Although it is generally accepted that oil is the most valuable natural resource
chemical raw materials, so far the main amount of oil and oil products
burns in internal combustion engines (gasoline), diesel engines and jet engines
engines (kerosene).
motor fuel. Octane number. Gasolines of various origins
behave differently in internal combustion engines.
In an effort to maximize engine power with small dimensions and
mass, they try to increase the compression ratio of the combustible mixture in the cylinder. However, in
high-speed four-stroke engines working with forced ignition,
in this case, premature ignition of the mixture sometimes occurs -
detonation. This reduces the power of the motor and accelerates its wear. This phenomenon
associated with the composition of liquid fuel, since hydrocarbons of different structures at
when used as a motor fuel, they behave differently. Worst
indicators - for paraffins of normal structure.
The standard for a combustible substance with a high detonation ability is taken
normal heptane. The more branched the carbon chain of the paraffin
hydrocarbon, the better its combustion in the cylinder proceeds and the greater the degree
compression of the combustible mixture can be achieved. As motor fuel standard
adopted 2, 2, 4-trimethylpentane (commonly referred to as isooctane) with good
antiknock properties. Composing in various proportions mixtures of this
octane with n-heptap, compare their behavior in the motor with the behavior of the subject
investigated gasoline, then they say that the latter has octane number 70
assumed to be zero).
One of the ways to improve the knock resistance of fuels for engines with
spark ignition is the application antiknock agents.
Antiknock agents are substances that are added to gasoline (not more than 0.5%) to
improvement of antidetopathic properties. Sufficiently effective antiknock
is an tetraethyl lead(TES) Pb (C2H5)4
However, gasoline from thermal power plants and its combustion products are very toxic. Currently
new antiknock agents based on manganese-organic compounds of the type
cyclopC5H5Mn (CO)5: they are less toxic and
have the best anti-knock properties. Adding these
antiknock to good grades of gasoline allows you to get fuel with
octane up to 135.
For rocket and diesel engines, on the contrary, fuels with
a normal chain of carbon atoms, having the lowest temperature
ignition. This characteristic is taken
evaluate in cetane numbers. A cetane number of 100 has a hydrocarbon
n-Sc, Hd4, and the cetap number 0 is 1-methylnaphthalene.
Synthesis of hydrocarbons from CO+H2. Flowing over finely crushed nickel
a mixture of carbon monoxide (II) and hydrogen at 250 ° C, you can get methane:
CO+3H2CH4+H2O
If this reaction is carried out at a pressure of 100-200 atm and a temperature up to 400°C,
a mixture is obtained, consisting mainly of oxygen-containing products,
among which alcohols predominate; this mixture was called schshpolom.
When using iron-cobalt catalysts and a temperature of 200 ° C,
mixture of alkanes syntin.
nCO + (2n + 1) H2 CnH2n + 2 + H2O
Sintin and synthol are products of large-scale organic synthesis and
are widely used as raw materials for many chemical industries.
Clathrates. Sintin and gasoline fractions of oil are composed of mixtures of hydrocarbons
normal structure and branched chains. Recently found effective
a method for separating organic compounds with normal chains and branched ones,
generally known as clathrate separation method. For
separation of hydrocarbons was used urea. Urea crystals
built in such a way that inside the crystals there are narrow hexagonal
channels. The diameter of these channels is such that it can pass through and linger inside them.
due to adsorption forces, only hydrocarbons of a normal structure. Therefore, when
treatment of a mixture of organic compounds with urea (or some other
compounds) substances with a normal chain of carbon atoms crystallize
together with it in the form of complexes. This method has, of course, a very large
the future is when more effective clathrate formers are found.
Characteristic chemical properties of hydrocarbons: alkanes, alkenes, dienes, alkynes, aromatic hydrocarbons
Alkanes
Alkanes are hydrocarbons in whose molecules the atoms are linked by single bonds and which correspond to the general formula $C_(n)H_(2n+2)$.
Homologous series of methane
As you already know, homologues are substances that are similar in structure and properties and differ by one or more $CH_2$ groups.
Limit hydrocarbons make up the homologous series of methane.
Isomerism and nomenclature
Alkanes are characterized by the so-called structural isomerism. Structural isomers differ from each other in the structure of the carbon skeleton. As you already know, the simplest alkane, which is characterized by structural isomers, is butane:
Let us consider in more detail for alkanes the basics of the IUPAC nomenclature:
1. Choice of the main circuit.
The formation of the name of a hydrocarbon begins with the definition of the main chain - the longest chain of carbon atoms in the molecule, which is, as it were, its basis.
2.
The atoms of the main chain are assigned numbers. The numbering of atoms of the main chain starts from the end closest to the substituent (structures A, B). If the substituents are at an equal distance from the end of the chain, then the numbering starts from the end at which there are more of them (structure B). If different substituents are at an equal distance from the ends of the chain, then the numbering starts from the end to which the older one is closer (structure G). The seniority of hydrocarbon substituents is determined by the order in which the letter with which their name begins follows in the alphabet: methyl (—$CH_3$), then propyl ($—CH_2—CH_2—CH_3$), ethyl ($—CH_2—CH_3$ ) etc.
Note that the name of the substitute is formed by replacing the suffix -an to suffix -silt in the name of the corresponding alkane.
3. Name formation.
Numbers are indicated at the beginning of the name - the numbers of carbon atoms at which the substituents are located. If there are several substituents at a given atom, then the corresponding number in the name is repeated twice, separated by commas ($2.2-$). After the number, a hyphen indicates the number of substituents ( di- two, three- three, tetra- four, penta- five) and the name of the deputy ( methyl, ethyl, propyl). Then without spaces and hyphens - the name of the main chain. The main chain is called as a hydrocarbon - a member of the homologous series of methane ( methane, ethane, propane, etc.).

The names of the substances whose structural formulas are given above are as follows:
- structure A: $2$ -methylpropane;
- Structure B: $3$ -ethylhexane;
- Structure B: $2,2,4$ -trimethylpentane;
- structure Г: $2$ -methyl$4$-ethylhexane.
Physical and chemical properties of alkanes
physical properties. The first four representatives of the homologous series of methane are gases. The simplest of them is methane - a colorless, tasteless and odorless gas (the smell of gas, upon smelling which you need to call $104$, is determined by the smell of mercaptans - sulfur-containing compounds specially added to methane used in household and industrial gas appliances in order to make people those near them could smell the leak).
Hydrocarbons of composition from $С_5Н_(12)$ to $С_(15)Н_(32)$ are liquids; heavier hydrocarbons are solids.
The boiling and melting points of alkanes gradually increase with increasing carbon chain length. All hydrocarbons are poorly soluble in water; liquid hydrocarbons are common organic solvents.
Chemical properties.
1. substitution reactions. The most characteristic of alkanes are free radical substitution reactions, during which a hydrogen atom is replaced by a halogen atom or some group.
Let us present the equations of the most typical reactions.
Halogenation:
$CH_4+Cl_2→CH_3Cl+HCl$.
In the case of an excess of halogen, chlorination can go further, up to the complete replacement of all hydrogen atoms by chlorine:
$CH_3Cl+Cl_2→HCl+(CH_2Cl_2)↙(\text"dichloromethane(methylene chloride)")$,
$CH_2Cl_2+Cl_2→HCl+(CHСl_3)↙(\text"trichloromethane(chloroform)")$,
$CHCl_3+Cl_2→HCl+(CCl_4)↙(\text"tetrachloromethane(carbon tetrachloride)")$.
The resulting substances are widely used as solvents and starting materials in organic synthesis.
2. Dehydrogenation (elimination of hydrogen). During the passage of alkanes over the catalyst ($Pt, Ni, Al_2O_3, Cr_2O_3$) at a high temperature ($400-600°C$), a hydrogen molecule is split off and an alkene is formed:
$CH_3—CH_3→CH_2=CH_2+H_2$
3. Reactions accompanied by the destruction of the carbon chain. All saturated hydrocarbons are burning with the formation of carbon dioxide and water. Gaseous hydrocarbons mixed with air in certain proportions can explode. The combustion of saturated hydrocarbons is a free radical exothermic reaction that has a very great importance when using alkanes as fuel:
$CH_4+2O_2→CO_2+2H_2O+880 kJ.$
In general, the combustion reaction of alkanes can be written as follows:
$C_(n)H_(2n+2)+((3n+1)/(2))O_2→nCO_2+(n+1)H_2O$
Thermal breakdown of hydrocarbons:
$C_(n)H_(2n+2)(→)↖(400-500°C)C_(n-k)H_(2(n-k)+2)+C_(k)H_(2k)$
The process proceeds according to the free radical mechanism. An increase in temperature leads to a homolytic rupture of the carbon-carbon bond and the formation of free radicals:
$R—CH_2CH_2:CH_2—R→R—CH_2CH_2+CH_2—R$.
These radicals interact with each other, exchanging a hydrogen atom, with the formation of an alkane molecule and an alkene molecule:
$R—CH_2CH_2+CH_2—R→R—CH=CH_2+CH_3—R$.
Thermal splitting reactions underlie the industrial process - hydrocarbon cracking. This process is the most important stage of oil refining.
When methane is heated to a temperature of $1000°C$, pyrolysis of methane begins - decomposition into simple substances:
$CH_4(→)↖(1000°C)C+2H_2$
When heated to a temperature of $1500°C$, the formation of acetylene is possible:
$2CH_4(→)↖(1500°C)CH=CH+3H_2$
4. Isomerization. When linear hydrocarbons are heated with an isomerization catalyst (aluminum chloride), substances with a branched carbon skeleton are formed:
5. Aromatization. Alkanes with six or more carbon atoms in the chain in the presence of a catalyst are cyclized to form benzene and its derivatives:

What is the reason that alkanes enter into reactions proceeding according to the free radical mechanism? All carbon atoms in alkane molecules are in the $sp^3$ hybridization state. The molecules of these substances are built using covalent nonpolar $C—C$ (carbon—carbon) bonds and weakly polar $C—H$ (carbon—hydrogen) bonds. They do not contain areas with high and low electron density, easily polarizable bonds, i.e. such bonds, the electron density in which can be shifted under the influence of external factors (electrostatic fields of ions). Therefore, alkanes will not react with charged particles, because bonds in alkane molecules are not broken by a heterolytic mechanism.
Alkenes
Unsaturated hydrocarbons include hydrocarbons containing multiple bonds between carbon atoms in molecules. Unlimited are alkenes, alkadienes (polyenes), alkynes. Cyclic hydrocarbons containing a double bond in the cycle (cycloalkenes), as well as cycloalkanes with a small number of carbon atoms in the cycle (three or four atoms) also have an unsaturated character. The property of unsaturation is associated with the ability of these substances to enter into addition reactions, primarily hydrogen, with the formation of saturated, or saturated, hydrocarbons - alkanes.
Alkenes are acyclic hydrocarbons containing in the molecule, in addition to single bonds, one double bond between carbon atoms and corresponding to the general formula $C_(n)H_(2n)$.
Its second name olefins- alkenes were obtained by analogy with unsaturated fatty acids (oleic, linoleic), the remains of which are part of liquid fats - oils (from lat. oleum- butter).
Homologous series of ethene
Unbranched alkenes make up the homologous series of ethene (ethylene):
$C_2H_4$ is ethene, $C_3H_6$ is propene, $C_4H_8$ is butene, $C_5H_(10)$ is pentene, $C_6H_(12)$ is hexene, etc.
Isomerism and nomenclature
For alkenes, as well as for alkanes, structural isomerism is characteristic. Structural isomers differ from each other in the structure of the carbon skeleton. The simplest alkene, which is characterized by structural isomers, is butene:

A special type of structural isomerism is the double bond position isomerism:
$CH_3—(CH_2)↙(butene-1)—CH=CH_2$ $CH_3—(CH=CH)↙(butene-2)—CH_3$
Almost free rotation of carbon atoms is possible around a single carbon-carbon bond, so alkane molecules can take on a wide variety of shapes. Rotation around the double bond is impossible, which leads to the appearance of another type of isomerism in alkenes - geometric, or cis-trans isomerism.

cis- isomers are different from trance- isomers by the spatial arrangement of fragments of the molecule (in this case, methyl groups) relative to the $π$-bond plane, and, consequently, by their properties.
Alkenes are isomeric to cycloalkanes (interclass isomerism), for example:

The nomenclature of alkenes developed by IUPAC is similar to the nomenclature of alkanes.
1. Choice of the main circuit.
The formation of the name of a hydrocarbon begins with the definition of the main chain - the longest chain of carbon atoms in a molecule. In the case of alkenes, the main chain must contain a double bond.
2. Atom numbering of the main chain.
The numbering of the atoms of the main chain starts from the end to which the double bond is closest. For example, the correct connection name is:

$5$-methylhexene-$2$, not $2$-methylhexene-$4$, as might be expected.
If it is impossible to determine the beginning of the numbering of atoms in the chain by the position of the double bond, then it is determined by the position of the substituents, just as for saturated hydrocarbons.

3. Name formation.
The names of alkenes are formed in the same way as the names of alkanes. At the end of the name indicate the number of the carbon atom at which the double bond begins, and the suffix indicating that the compound belongs to the class of alkenes - -en.
For example:

Physical and chemical properties of alkenes
physical properties. The first three representatives of the homologous series of alkenes are gases; substances of the composition $C_5H_(10)$ - $C_(16)H_(32)$ are liquids; higher alkenes are solids.
The boiling and melting points naturally increase with an increase in the molecular weight of the compounds.
Chemical properties.
Addition reactions. Recall that a distinctive feature of the representatives unsaturated hydrocarbons- alkenes is the ability to enter into addition reactions. Most of these reactions proceed by the mechanism
1. hydrogenation of alkenes. Alkenes are able to add hydrogen in the presence of hydrogenation catalysts, metals - platinum, palladium, nickel:
$CH_3—CH_2—CH=CH_2+H_2(→)↖(Pt)CH_3—CH_2—CH_2—CH_3$.
This reaction proceeds at atmospheric and elevated pressure and does not require high temperature, because is exothermic. With an increase in temperature on the same catalysts, the reverse reaction, dehydrogenation, can occur.
2. Halogenation (addition of halogens). The interaction of an alkene with bromine water or a solution of bromine in an organic solvent ($CCl_4$) leads to a rapid discoloration of these solutions as a result of the addition of a halogen molecule to the alkene and the formation of dihalogen alkanes:
$CH_2=CH_2+Br_2→CH_2Br—CH_2Br$.
3.
$CH_3-(CH)↙(propene)=CH_2+HBr→CH_3-(CHBr)↙(2-bromopropene)-CH_3$
This reaction is subject to Markovnikov's rule:
When a hydrogen halide is added to an alkene, hydrogen is attached to a more hydrogenated carbon atom, i.e. the atom at which there are more hydrogen atoms, and the halogen - to the less hydrogenated one.
Hydration of alkenes leads to the formation of alcohols. For example, the addition of water to ethene underlies one of the industrial methods for producing ethyl alcohol:
$(CH_2)↙(ethene)=CH_2+H_2O(→)↖(t,H_3PO_4)CH_3-(CH_2OH)↙(ethanol)$
Note that a primary alcohol (with a hydroxo group at the primary carbon) is formed only when ethene is hydrated. When propene or other alkenes are hydrated, secondary alcohols are formed.

This reaction also proceeds in accordance with Markovnikov's rule - the hydrogen cation is attached to the more hydrogenated carbon atom, and the hydroxo group to the less hydrogenated one.
5. Polymerization. A special case of addition is the polymerization reaction of alkenes:
$nCH_2(=)↙(ethene)CH_2(→)↖(UV light,R)(...(-CH_2-CH_2-)↙(polyethylene)...)_n$
This addition reaction proceeds by a free radical mechanism.
6. Oxidation reaction.
Like any organic compounds, alkenes burn in oxygen to form $СО_2$ and $Н_2О$:
$CH_2=CH_2+3O_2→2CO_2+2H_2O$.
In general:
$C_(n)H_(2n)+(3n)/(2)O_2→nCO_2+nH_2O$
Unlike alkanes, which are resistant to oxidation in solutions, alkenes are easily oxidized by the action of potassium permanganate solutions. In neutral or alkaline solutions, alkenes are oxidized to diols (dihydric alcohols), and hydroxyl groups are attached to those atoms between which a double bond existed before oxidation:

Alkadienes (diene hydrocarbons)
Alkadienes are acyclic hydrocarbons containing in the molecule, in addition to single bonds, two double bonds between carbon atoms and corresponding to the general formula $C_(n)H_(2n-2)$.
Depending on the relative position double bonds, there are three types of dienes:
- alkadienes with cumulated arrangement of double bonds:
- alkadienes with conjugated double bonds;
$CH_2=CH—CH=CH_2$;
- alkadienes with isolated double bonds
$CH_2=CH—CH_2—CH=CH_2$.
All three types of alkadienes differ significantly from each other in structure and properties. The central carbon atom (an atom that forms two double bonds) in alkadienes with cumulated bonds is in the $sp$-hybridization state. It forms two $σ$-bonds lying on the same straight line and directed in opposite directions, and two $π$-bonds lying in perpendicular planes. $π$-bonds are formed due to unhybridized p-orbitals of each carbon atom. The properties of alkadienes with isolated double bonds are very specific, because conjugated $π$-bonds significantly affect each other.
p-Orbitals forming conjugated $π$-bonds make up practically a single system (it is called a $π$-system), because p-orbitals of neighboring $π$-bonds partially overlap.
Isomerism and nomenclature
Alkadienes are characterized by both structural isomerism and cis- and trans-isomerism.
Structural isomerism.
— isomerism of the carbon skeleton:

— isomerism of the position of multiple bonds:
$(CH_2=CH—CH=CH_2)↙(butadiene-1,3)$ $(CH_2=C=CH—CH_3)↙(butadiene-1,2)$
cis-, trans- isomerism (spatial and geometric)
For example:
Alkadienes are isomeric compounds of the classes of alkynes and cycloalkenes.
When forming the name of the alkadiene, the numbers of double bonds are indicated. The main chain must necessarily contain two multiple bonds.
For example:

Physical and chemical properties of alkadienes
physical properties.
Under normal conditions, propandien-1,2, butadiene-1,3 are gases, 2-methylbutadiene-1,3 is a volatile liquid. Alkadienes with isolated double bonds (the simplest of them is pentadiene-1,4) are liquids. Higher dienes are solids.
Chemical properties.
The chemical properties of alkadienes with isolated double bonds differ little from those of alkenes. Alkadienes with conjugated bonds have some special features.
1. Addition reactions. Alkadienes are capable of adding hydrogen, halogens, and hydrogen halides.
A feature of addition to alkadienes with conjugated bonds is the ability to attach molecules both in positions 1 and 2, and in positions 1 and 4.

The ratio of the products depends on the conditions and method of carrying out the corresponding reactions.
2.polymerization reaction. The most important property dienes is the ability to polymerize under the influence of cations or free radicals. The polymerization of these compounds is the basis of synthetic rubbers:
$nCH_2=(CH—CH=CH_2)↙(butadiene-1,3)→((... —CH_2—CH=CH—CH_2— ...)_n)↙(\text"synthetic butadiene rubber")$ .
The polymerization of conjugated dienes proceeds as 1,4-addition.
In this case, the double bond turns out to be central in the link, and the elementary link, in turn, can take both cis-, and trance- configuration.

Alkynes
Alkynes are acyclic hydrocarbons containing in the molecule, in addition to single bonds, one triple bond between carbon atoms and corresponding to the general formula $C_(n)H_(2n-2)$.
Homologous series of ethine
Unbranched alkynes make up the homologous series of ethyne (acetylene):
$C_2H_2$ - ethyne, $C_3H_4$ - propyne, $C_4H_6$ - butyne, $C_5H_8$ - pentine, $C_6H_(10)$ - hexine, etc.
Isomerism and nomenclature
For alkynes, as well as for alkenes, structural isomerism is characteristic: isomerism of the carbon skeleton and isomerism of the position of the multiple bond. The simplest alkyne, which is characterized by structural isomers of the multiple bond position of the alkyne class, is butyne:
$CH_3—(CH_2)↙(butyn-1)—C≡CH$ $CH_3—(C≡C)↙(butyn-2)—CH_3$
The isomerism of the carbon skeleton in alkynes is possible, starting from pentyn:

Since the triple bond assumes a linear structure of the carbon chain, the geometric ( cis-, trans-) isomerism is not possible for alkynes.
The presence of a triple bond in hydrocarbon molecules of this class is reflected by the suffix -in, and its position in the chain - the number of the carbon atom.
For example:

Alkynes are isomeric compounds of some other classes. So, chemical formula$С_6Н_(10)$ have hexine (alkyne), hexadiene (alkadiene) and cyclohexene (cycloalkene):

Physical and chemical properties of alkynes
physical properties. The boiling and melting points of alkynes, as well as alkenes, naturally increase with an increase in the molecular weight of the compounds.
Alkynes have a specific smell. They are more soluble in water than alkanes and alkenes.
Chemical properties.
Addition reactions. Alkynes are unsaturated compounds and enter into addition reactions. Basically, these are reactions. electrophilic addition.
1. Halogenation (addition of a halogen molecule). Alkyne is able to attach two halogen molecules (chlorine, bromine):
$CH≡CH+Br_2→(CHBr=CHBr)↙(1,2-dibromoethane),$
$CHBr=CHBr+Br_2→(CHBr_2-CHBr_2)↙(1,1,2,2-tetrabromoethane)$
2. Hydrohalogenation (addition of hydrogen halide). The addition reaction of hydrogen halide, proceeding according to the electrophilic mechanism, also proceeds in two stages, and at both stages the Markovnikov rule is fulfilled:
$CH_3-C≡CH+Br→(CH_3-CBr=CH_2)↙(2-bromopropene),$
$CH_3-CBr=CH_2+HBr→(CH_3-CHBr_2-CH_3)↙(2,2-dibromopropane)$
3. Hydration (addition of water). Of great importance for the industrial synthesis of ketones and aldehydes is the water addition reaction (hydration), which is called Kucherov's reaction:
4. hydrogenation of alkynes. Alkynes add hydrogen in the presence of metal catalysts ($Pt, Pd, Ni$):
$R-C≡C-R+H_2(→)↖(Pt)R-CH=CH-R,$
$R-CH=CH-R+H_2(→)↖(Pt)R-CH_2-CH_2-R$
Since the triple bond contains two reactive $π$ bonds, alkanes add hydrogen in steps:
1) trimerization.
When ethyne is passed over activated carbon, a mixture of products is formed, one of which is benzene:

2) dimerization.
In addition to trimerization of acetylene, its dimerization is also possible. Under the action of monovalent copper salts, vinylacetylene is formed:
$2HC≡CH→(HC≡C-CH=CH_2)↙(\text"butene-1-yn-3(vinylacetylene)")$
This substance is used to produce chloroprene:
$HC≡C-CH=CH_2+HCl(→)↖(CaCl)H_2C=(CCl-CH)↙(chloroprene)=CH_2$
polymerization of which produces chloroprene rubber:
$nH_2C=CCl-CH=CH_2→(...-H_2C-CCl=CH-CH_2-...)_n$
Alkyne oxidation.
Ethine (acetylene) burns in oxygen with the release of a very large amount of heat:
$2C_2H_2+5O_2→4CO_2+2H_2O+2600kJ$ The action of an oxy-acetylene torch is based on this reaction, the flame of which has a very high temperature (more than $3000°C$), which makes it possible to use it for cutting and welding metals.
In air, acetylene burns with a smoky flame, because. the carbon content in its molecule is higher than in the molecules of ethane and ethene.
Alkynes, like alkenes, decolorize acidified solutions of potassium permanganate; in this case, the destruction of the multiple bond occurs.
Reactions characterizing the main methods for obtaining oxygen-containing compounds
1. Hydrolysis of haloalkanes. You already know that the formation of halokenalkanes in the interaction of alcohols with hydrogen halides is a reversible reaction. Therefore, it is clear that alcohols can be obtained by hydrolysis of haloalkanes- reactions of these compounds with water:
$R-Cl+NaOH(→)↖(H_2O)R-OH+NaCl+H_2O$
Polyhydric alcohols can be obtained by hydrolysis of haloalkanes containing more than one halogen atom in the molecule. For example:
2. Hydration of alkenes- the addition of water to the $π$-bond of the alkene molecule - is already familiar to you, for example:
$(CH_2=CH_2)↙(ethene)+H_2O(→)↖(H^(+))(C_2H_5OH)↙(ethanol)$
Hydration of propene leads, in accordance with Markovnikov's rule, to the formation of a secondary alcohol - propanol-2:

3. Hydrogenation of aldehydes and ketones. You already know that the oxidation of alcohols under mild conditions leads to the formation of aldehydes or ketones. Obviously, alcohols can be obtained by hydrogenation (hydrogen reduction, hydrogen addition) of aldehydes and ketones:

4. Alkene oxidation. Glycols, as already noted, can be obtained by oxidizing alkenes with an aqueous solution of potassium permanganate. For example, ethylene glycol (ethanediol-1,2) is formed during the oxidation of ethylene (ethene):
$CH_2=CH_2+[O]+H_2O(→)↖(KMnO_4)HO-CH_2-CH_2-OH$
5. Specific methods for obtaining alcohols. Some alcohols are obtained in ways characteristic only of them. Thus, methanol is produced in industry by the interaction of hydrogen with carbon monoxide (II) (carbon monoxide) at elevated pressure and high temperature on the surface of the catalyst (zinc oxide):
$CO+2H_2(→)↖(t,p,ZnO)CH_3-OH$
The mixture required for this reaction carbon monoxide and hydrogen, also called synthesis gas ($CO + nH_2O$), is obtained by passing water vapor over hot coal:
$C+H_2O(→)↖(t)CO+H_2-Q$
6. Fermentation of glucose. This method of obtaining ethyl (wine) alcohol has been known to man since ancient times:
$(C_6H_(12)O_6)↙(glucose)(→)↖(yeast)2C_2H_5OH+2CO_2$
Methods for obtaining aldehydes and ketones
Aldehydes and ketones can be obtained oxidation or alcohol dehydrogenation. Once again, we note that aldehydes can be obtained during the oxidation or dehydrogenation of primary alcohols, and ketones can be obtained from secondary alcohols:

Kucherov's reaction. From acetylene, as a result of the hydration reaction, acetaldehyde is obtained, from acetylene homologs - ketones:

When heated calcium or barium salts carboxylic acids form a ketone and a metal carbonate:

Methods for obtaining carboxylic acids
Carboxylic acids can be obtained by oxidation of primary alcohols of aldehydes:

Aromatic carboxylic acids are formed during the oxidation of benzene homologues:

Hydrolysis of various carboxylic acid derivatives also results in acids. So, during the hydrolysis of an ester, an alcohol and a carboxylic acid are formed. As mentioned above, acid-catalyzed esterification and hydrolysis reactions are reversible:
Hydrolysis of an ester by the action of aqueous solution alkali proceeds irreversibly, in this case, not an acid is formed from the ester, but its salt.