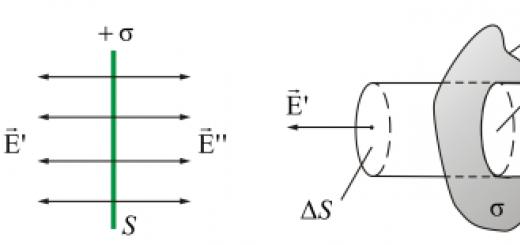
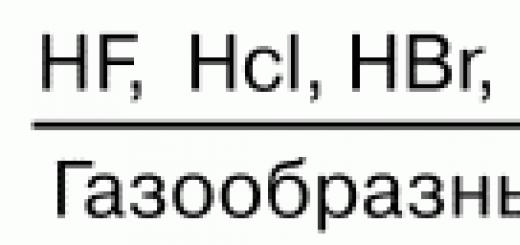When preparing solutions of percentage concentration, the substance is weighed on a techno-chemical balance, and liquids are measured with a measuring cylinder. Therefore, a hitch! substances are calculated with an accuracy of 0.1 g, and the volume of 1 liquid with an accuracy of 1 ml.
Before proceeding with the preparation of the solution, | | it is necessary to make a calculation, i.e., calculate the amount of solute and solvent to prepare a certain amount of a solution of a given concentration.
CALCULATIONS IN THE PREPARATION OF SALT SOLUTIONS
Example 1. It is necessary to prepare 500 g of a 5% solution of potassium nitrate. 100 g of such a solution contains 5 g of KN0 3; 1 We make up the proportion:
100 g solution-5 g KN0 3
500 » 1 - X» KN0 3
5-500 "_ x \u003d -jQg- \u003d 25 g.
Water should be taken 500-25 = 475 ml.
Example 2. It is necessary to prepare 500 g of a 5% CaCl solution from CaCl 2 -6H 2 0 salt. First, we calculate for anhydrous salt.
100 g solution - 5 g CaCl 2 500 "" - X "CaCl 2 5-500 _ x = 100 = 25 g -
The molar mass of CaCl 2 \u003d 111, the molar mass of CaCl 2 - 6H 2 0 \u003d 219 *. Therefore, 219 g of CaCl 2 -6H 2 0 contain 111 g of CaCl 2 . We make a proportion:
219 g CaC1 2 -6H 2 0-111 g CaC1 2
X "CaCl 2 -6H 2 0-26" CaCI,
219-25 x \u003d -jjj- \u003d 49.3 g.
The amount of water is 500-49.3=450.7 g, or 450.7 ml. Since water is measured with a graduated cylinder, tenths of a milliliter are not taken into account. Therefore, you need to measure 451 ml of water.
CALCULATIONS IN THE PREPARATION OF ACID SOLUTIONS
When preparing acid solutions, it must be taken into account that concentrated acid solutions are not 100% and contain water. In addition, the required amount of acid is not weighed, but measured with a graduated cylinder.
Example 1. It is necessary to prepare 500 g of a 10% hydrochloric acid solution, based on the available 58% acid, the density of which is d=l,19.
1. Find the amount of pure hydrogen chloride that should be in the prepared acid solution:
100 g solution -10 g HC1 500 » » - X » HC1 500-10 * = 100 = 50 g -
* To calculate the solutions of the percentage concentration of the mole, the mass is rounded to whole numbers.
2. Find the number of grams of concentrated)
acid, which will contain 50 g of HC1:
100 g acid-38 g HC1 X » » -50 » HC1 100 50
X gg—"= 131.6 G.
3. Find the volume that this amount occupies 1
acids:
V--— 131 ‘ 6 110 6 sch
4. The amount of solvent (water) is 500-;
-131.6 = 368.4 g or 368.4 ml. Since the necessary co-
the amount of water and acid is measured with a measuring cylinder
rum, then tenths of a milliliter are not taken into account
ut. Therefore, to prepare 500 g of a 10% solution
hydrochloric acid, you need to take 111 ml of hydrochloric acid I
acids and 368 ml of water.
Example 2 Usually, in calculations for the preparation of acids, standard tables are used that indicate the percentage of an acid solution, the density of a given solution at a certain temperature, and the number of grams of this acid contained in 1 liter of a solution of a given concentration (see Annex V). In this case, the calculation is simplified. The amount of prepared acid solution can be calculated for a certain volume.
For example, you need to prepare 500 ml of a 10% hydrochloric acid solution, based on a concentrated 38% j solution. According to the tables, we find that a 10% hydrochloric acid solution contains 104.7 g of HC1 in 1 liter of solution. We need to prepare 500 ml of I, therefore, the solution should be 104.7: 2 \u003d 52.35 g of HO.
Calculate how much you need to take concentrated I acids. According to the table, 1 liter of concentrated HC1 contains 451.6 g of HC1. We make up the proportion: 1000 ml-451.6 g of HC1 X » -52.35 » HC1
1000-52.35 x \u003d 451.6 \u003d "5 ml.
The amount of water is 500-115 = 385 ml.
Therefore, to prepare 500 ml of a 10% hydrochloric acid solution, you need to take 115 ml of a concentrated HC1 solution and 385 ml of water.
Soldering acid is a flux that is in a special category because it is highly corrosive to the materials it works with. This substance is distributed mainly in liquid form, regardless of its concentration. Sometimes a diluted variety may be sold, or a concentrated substance that can be diluted on its own. In addition, you can still try to make soldering acid with your own hands.

All properties of the material determine the scope of its application. It is intended more for heavily contaminated metals, which quickly form oxides or have rust residues on the surface. Due to the high activity of the material is dangerous for contact with the skin and the surface of the mucous membranes. You need to know the rules for using acid before you start working with it.
The technology of how to make soldering acid at home suggests that the result should be a substance that would have properties that best correspond to GOST 23178-78. This will help improve the quality of the flux in order to get reliable joints. The main thing is that the properties of the acid appear after application, since the flux on the metal not only removes fatty films and oxides, but also prevents their re-formation. It is also worth noting the best spreading of the solder over the surface and a high level of adhesion with the base material.
Physico-chemical properties and composition
Before you make soldering acid, you should familiarize yourself with the composition of the material. This substance includes:
- hydrochloric acid;
- ammonium chloride;
- zinc chloride;
- Water deionized;
- Wetting agent.
Soldering acid at home may have other components in its composition. The main thing is to achieve the mandatory properties that this flux possesses. First, there must be a high activity of the material. Rapid interaction with the elements makes the environment aggressive and destroys almost all harmful substances that interfere with normal soldering. This has the side effect that small metal parts can be damaged by contact with the acid. Active soldering fat also has similar properties.
The acid emits a peculiar odor and is harmful to health when a person inhales its vapors. Thus, during work, a respirator should be used, and the room in which this all takes place should be well ventilated. It is required to prevent the flux from getting on hands, eyes, and other surfaces, except for the workpiece itself and solder.
Necessary tools and materials for manufacturing
It should be understood that soldering acid at home will have a slightly different composition, which in turn makes it easier to manufacture. For its preparation, the following materials and tools are required:
- A jar or other container for cooking and mixing (preferably glass);
- Granular zinc or instead of it, cups from old batteries that contain this element can be used;
- Water, which serves to dilute the concentrate;
- Concentrated hydrochloric acid, which is the main element and can dissolve additional impurities.

Do-it-yourself technology for creating acid for soldering
First of all, a laboratory container is prepared, which is a glass jar, or other porcelain and ceramic container. Zinc or battery residue should be placed in it. Only after the additives are placed in the container is the hydrochloric acid concentrate placed. It must be poured very carefully, since if it gets on your hand, you can get a chemical burn. The total liquid level in the tank should not exceed ¾ of the total volume.

The proportions of the substance, if there are accurate measuring instruments, should look like this - 412 g of zinc is required per liter of hydrochloric acid. Naturally, small deviations are possible, but they should not be too high.
The next step on how to prepare soldering acid is to wait for the end of the reaction. When acid and zinc come into contact, the metal begins to dissolve. During dissolution, hydrogen is released, causing bubbles to form in the liquid.

Also, the liquid becomes more transparent. After everything is over, the resulting substance should be poured into another container, which is tightly closed. You can buy all the materials without any problems in stores that sell chemical reagents. If you use batteries, then almost any type of "AAA" and "AA" will do.
If you are looking for a non-solo concentrated material, but need to make something weaker that would not have a high level of aggressiveness, then you can add water to reduce the concentration. This must also be done very carefully so as not to splash the liquid. Proportions can be selected independently, depending on the features of soldering.
How to prepare soldering acid at home
First of all, you need to pay attention to security measures, as this is a very dangerous business. In production at enterprises, everything is done in special cabinets, where reagents are mixed under an exhaust hood and in protected places. At home, it is imperative to use personal protective equipment that will help protect the skin, eyes, respiratory organs and others. The dissolution process is best done outdoors on outdoors or provide good ventilation. This is necessary due to the fact that hydrogen is actively released into the air. There should also be a source of water nearby so that you can wash the damaged area of skin if an accident occurs. It is advisable to have running water from the tap, preferably cold, as this will slightly reduce the level of pain.
If the substance was spilled on any surface, then it can be washed off with a solution of alkali and water. Do not forget about the proper storage of the material, the container should be airtight, and everything should be stored in a cool, dark place. Outsiders who do not know should not have access to it. For the flux, pure hydrochloric acid is sometimes used, without the addition of zinc impurities, and also not diluted with water. This flux is most often used for iron materials.
The hydrochloric acid coming from the plant can have different concentrations, so it is necessary to calculate the amount of water and acid using table 6.2
Table 6.2
|
denseHClat 15 about С, kg/m 3 |
masses. shareHCl, % |
weight fractionHClkg/l |
denseHClat 15 about С, kg/m 3 |
masses. shareHCl, % |
weight fractionHClkg/l |
The amount of commercial acid in volume units required to obtain 1 m 3 of a working solution of a given concentration is determined by the formula:
V T \u003d n (r Z - 1000) / (r T - 1000) (5.2)
where n is the number of cubic meters of solution;
V T - the volume of commercial acid, m 3;
r t - commercial acid density, kg/m 3 ;
r З - the given density of the finished solution, kg / m 3, which is taken from table 6.2, based on the percentage mass content of HCl in the solution.
Example. Prepare 35 m 3 of a 12% HCl solution, if the density of the commercial acid is 1150 kg / m 3. According to table 6.2, we find that the density of a 12% HCl solution is 1060 kg / m 3. Then
V T \u003d 35 (1060 - 1000) / (1150 - 1000) \u003d 14 m 3
The volume of water for preparing the solution is 35 - 14 \u003d 21 m 3. Let's check the calculation results:
r W \u003d (14 × 1150 + 21 × 1000) / 35 \u003d 1060 kg / m 3
Equipment for acid treatment of wells
To treat the formation with acid, a set of equipment is used, which includes fittings for the wellhead (1AU - 700, 2AU - 700), a pump unit for injecting acid into the well, a tank truck for transporting acid and chemicals, a manifold for connecting the tank truck with the pump unit and with mouth fittings.
During hydrochloric acid treatment, the concentration of acid in the solution is 8-20%, depending on the treated rocks. If the concentration of HCl is higher than the recommended one, the pipes of the wellhead and downhole equipment are destroyed, and if it is lower, the efficiency of the treatment of the bottomhole zone decreases.
To protect pipes, tanks, pumps, pipelines, wellhead and downhole equipment from the corrosive effects of acid, inhibitors are added to the solution: formalin (0.6%), unicol (0.3 - 0.5%), reagent I-1-A ( 0.4%) and catapin A (0.1%).
To prevent the precipitation of iron oxides that clog the pores of the formation, stabilizers are used, which are used as acetic (0.8-1.6%) and hydrofluoric (1-2%) acids from the volume of diluted hydrochloric acid.
The HCl solution is prepared as follows: a calculated volume of water is poured into the container, an inhibitor is added to it, then a stabilizer and a reaction retarder - a DS preparation in an amount of 1 - 1.5% of the volume of the acid solution. After thorough mixing of the solution, the calculated volume of concentrated HCl is added last.
The fields use acid injection into the formation under pressure, acid baths to clean the bottom surface from contaminating deposits (cement, mud, resins, paraffin), as well as injection of a hot acid solution, which is heated due to the exothermic reaction between HCl and magnesium.
To transport the solution of inhibited HCl and inject it into the reservoirs, special units Azinmash - 30A, automatic transmission - 500, KP - 6.5 are used. The Azinmash - 30A unit is mounted on the chassis of a KrAZ - 257 vehicle. The unit consists of a three-plunger horizontal single-acting pump 5NK - 500 driven by a propulsion engine through a power take-off, a manifold, rubber-lined tanks main (6-10 m 3) and on a trailer (6 m 3).
Preparation of solutions. The solution is called homogeneous mixtures two or more substances. The concentration of a solution is expressed in different ways:
in weight percent, i.e. by the number of grams of the substance contained in 100 g of the solution;
in volume percent, i.e. by the number of volume units (ml) of the substance in 100 ml of solution;
molarity, i.e. the number of gram-moles of a substance in 1 liter of solution (molar solutions);
normality, i.e. the number of gram equivalents of a solute in 1 liter of solution.
Solutions of percentage concentration. Percentage solutions are prepared as approximate, while the sample of the substance is weighed on technochemical scales, and the volumes are measured with measuring cylinders.
Several methods are used to prepare percentage solutions.
Example. It is necessary to prepare 1 kg of a 15% sodium chloride solution. How much salt is needed for this? The calculation is carried out according to the proportion:
Therefore, water for this must be taken 1000-150 \u003d 850 g.
In those cases when it is necessary to prepare 1 liter of a 15% sodium chloride solution, the required amount of salt is calculated in a different way. According to the reference book, the density of this solution is found and, multiplying it by a given volume, the mass of the required amount of solution is obtained: 1000-1.184 \u003d 1184 g.
Then follows:
Therefore, the required amount of sodium chloride is different for the preparation of 1 kg and 1 liter of solution. In cases where solutions are prepared from reagents containing crystallization water, it should be taken into account when calculating the required amount of the reagent.
Example. It is necessary to prepare 1000 ml of a 5% solution of Na2CO3 with a density of 1.050 from a salt containing water of crystallization (Na2CO3-10H2O)
The molecular weight (weight) of Na2CO3 is 106 g, molecular mass(weight) Na2CO3-10H2O is equal to 286 g, from here the required amount of Na2CO3-10H2O is calculated to prepare a 5% solution:
Solutions are prepared by dilution method as follows.
Example. It is necessary to prepare 1 l of a 10% HCl solution from an acid solution with a relative density of 1.185 (37.3%). The relative density of a 10% solution is 1.047 (according to the reference table), therefore, the mass (weight) of 1 liter of such a solution is 1000X1.047 \u003d 1047 g. This amount of solution should contain pure hydrogen chloride
To determine how much 37.3% acid needs to be taken, we make up the proportion:

When preparing solutions by diluting or mixing two solutions, the diagonal scheme method or the "rule of the cross" is used to simplify calculations. At the intersection of two lines, the given concentration is written, and at both ends on the left is the concentration of the initial solutions, for the solvent it is equal to zero.