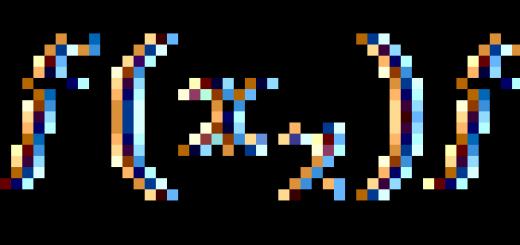As you know, many substances in nature can be in three states of aggregation: solid, liquid and gaseous.
The doctrine of the properties of matter in various states of aggregation is based on ideas about the atomic and molecular structure of the material world. The molecular-kinetic theory of the structure of matter (MKT) is based on three main provisions:
- All substances are made up of tiny particles (molecules, atoms, elementary particles), between which there are gaps;
- the particles are in continuous thermal motion;
- between the particles of matter there are forces of interaction (attraction and repulsion); the nature of these forces is electromagnetic.
This means that the state of aggregation of matter depends on relative position molecules, the distance between them, the forces of interaction between them and the nature of their movement.
The interaction of particles of matter in the solid state is most pronounced. The distance between molecules is approximately equal to their own sizes. This leads to a sufficiently strong interaction, which practically deprives the particles of the opportunity to move: they oscillate around a certain equilibrium position. They retain their shape and volume.
The properties of liquids are also explained by their structure. Particles of matter in liquids interact less intensively than in solids, and therefore they can change their location abruptly - liquids do not retain their shape - they are fluid. Liquids retain volume.
A gas is a collection of molecules moving randomly in all directions independently of each other. Gases do not have their own shape, they occupy the entire volume provided to them and are easily compressed.
There is another state of matter - plasma. Plasma is a partially or fully ionized gas in which the densities of positive and negative charges are almost the same. When heated sufficiently, any substance evaporates, turning into a gas. If the temperature is increased further, the process of thermal ionization will sharply increase, i.e., the gas molecules will begin to decompose into their constituent atoms, which then turn into ions.
Ideal gas model. Relationship between pressure and average kinetic energy.
To clarify the patterns that govern the behavior of matter in gaseous state, an idealized model of real gases is considered - an ideal gas. This is a gas whose molecules are considered as material points, which do not interact with each other at a distance, but interact with each other and with the walls of the vessel during collisions.
Ideal gas – it is a gas, the interaction between the molecules of which is negligible. (Ec>>Ep)
An ideal gas is a model invented by scientists to understand the gases that we observe in nature in reality. It may not describe any gas. Not applicable when the gas is highly compressed when the gas becomes liquid. Real gases behave like ideal gases when the average distance between molecules is many times greater than their sizes, i.e. at sufficiently high pressures.
Ideal gas properties:
- the distance between the molecules is much larger than the size of the molecules;
- gas molecules are very small and are elastic balls;
- the forces of attraction tend to zero;
- interactions between gas molecules occur only during collisions, and collisions are considered to be absolutely elastic;
- the molecules of this gas move randomly;
- the movement of molecules according to Newton's laws.
The state of a certain mass of a gaseous substance is characterized by mutually dependent physical quantities called state parameters. These include volumeV, pressurepand temperatureT.
Gas volume denoted V. Volume gas always coincides with the volume of the vessel that it occupies. SI unit of volume m 3.
Pressure– physical quantity, equal to the ratio strengthFacting on a surface element perpendicular to it, to the areaSthis element.
p = F/ S Unit of pressure in SI pascal[Pa]
Until now, off-system units of pressure have been used:
technical atmosphere 1 at = 9.81-104 Pa;
physical atmosphere 1 atm = 1.013-105 Pa;
millimeters of mercury 1 mmHg article = 133 Pa;
1 atm = = 760 mmHg Art. = 1013 hPa.
How is gas pressure generated? Each gas molecule, hitting the wall of the vessel in which it is located, acts on the wall with a certain force for a short period of time. As a result of random impacts on the wall, the force from all molecules per unit area of the wall changes rapidly with time relative to some (average) value.
Gas pressurearises as a result of chaotic impacts of molecules on the walls of the vessel in which the gas is located.
Using the ideal gas model, one can calculate gas pressure on the vessel wall.
In the process of interaction of a molecule with the vessel wall, forces arise between them that obey Newton's third law. As a result, the projection υ x velocity of the molecule perpendicular to the wall changes its sign to the opposite, and the projection υ y velocity parallel to the wall remains unchanged.
Instruments that measure pressure are called manometers. Pressure gauges record the time-averaged pressure force per unit area of its sensitive element (membrane) or other pressure receiver.

Liquid manometers:
- open - for measuring small pressures above atmospheric
- closed - for measuring small pressures below atmospheric, i.e. small vacuum


Metal pressure gauge- to measure high pressures.
Its main part is a curved tube A, the open end of which is soldered to the tube B, through which gas flows, and the closed end is connected to the arrow. Gas enters through the cock and tube B into tube A and unbends it. The free end of the tube, moving, drives the transmission mechanism and the arrow. The scale is graduated in units of pressure.


The basic equation of the molecular-kinetic theory of an ideal gas.
The basic equation of the MKT: the pressure of an ideal gas is proportional to the product of the mass of the molecule, the concentration of the molecules, and the mean square of the speed of the molecules
p= 1/3m 0· n v 2
m 0 is the mass of one gas molecule;
n = N/V is the number of molecules per unit volume, or the concentration of molecules;
v 2 - root mean square speed of molecules.
Since the average kinetic energy forward movement molecules E \u003d m 0 *v 2 /2, then multiplying the main MKT equation by 2, we get p \u003d 2/3 n (m 0 v 2) / 2 \u003d 2/3 E n
p = 2/3 E n
The gas pressure is equal to 2/3 of the average kinetic energy of the translational motion of molecules contained in a unit volume of gas.
Since m 0 n = m 0 N/V = m/V = ρ, where ρ is the gas density, we have p= 1/3 ρv 2
United gas law.
Macroscopic quantities that uniquely characterize the state of a gas are calledthermodynamic parameters of the gas.
The most important thermodynamic parameters of a gas are itsvolumeV, pressure p and temperature T.
Any change in the state of a gas is calledthermodynamic process.
In any thermodynamic process, the gas parameters that determine its state change.
The ratio between the values of certain parameters at the beginning and end of the process is calledgas law.
The gas law expressing the relationship between all three gas parameters is calledunified gas law.
p = nkT
Ratio p = nkT which relates the pressure of a gas to its temperature and concentration of molecules, was obtained for the model of an ideal gas, the molecules of which interact with each other and with the walls of the vessel only during elastic collisions. This ratio can be written in another form, establishing a relationship between the macroscopic parameters of the gas - the volume V, pressure p, temperature T and the amount of matter ν. To do this, you need to use the equalities
where n is the concentration of molecules, N is total number molecules, V is the volume of gas
Then we get either
Since at a constant mass of gas N remains unchanged, then Nk - constant number, means
At a constant mass of gas, the product of volume and pressure, divided by the absolute temperature of the gas, is the same value for all states of this mass of gas.
The equation establishing the relationship between pressure, volume and temperature of a gas was obtained in the middle of the 19th century by the French physicist B. Clapeyron and is often called Claiperon equation.
The Claiperon equation can be written in another form.
p = nkt,
given that

Here N is the number of molecules in the vessel, ν is the amount of substance, N A is the Avogadro constant, m is the mass of gas in the vessel, M is the molar mass of the gas. As a result, we get:

The product of the Avogadro constant N A byBoltzmann's constantk is called universal (molar) gas constant and is marked with the letter R.
Her numerical value in SI R= 8.31 J/mol K
Ratio
![]()
called ideal gas equation of state.
In the form we received, it was first recorded by D. I. Mendeleev. Therefore, the equation of state of the gas is called the Clapeyron–Mendeleev equation.`
For one mole of any gas, this ratio takes the form: pV=RT
Let's install physical meaning molar gas constant. Suppose that in a certain cylinder under the piston at a temperature E there is 1 mole of gas, the volume of which is V. If the gas is heated isobarically (at constant pressure) by 1 K, the piston will rise to a height Δh, and the gas volume will increase by ΔV.
Let's write the equation pV=RT for heated gas: p (V + ΔV) = R (T + 1)
and subtract from this equation the equation pV=RT corresponding to the state of the gas before heating. We get pΔV = R
ΔV = SΔh, where S is the base area of the cylinder. Substitute in the resulting equation:
pS = F is the pressure force.
We get FΔh = R, and the product of the force and the displacement of the piston FΔh = A is the work of displacement of the piston, performed by this force against external forces during the expansion of the gas.
Thus, R = A.
The universal (molar) gas constant is numerically equal to the work that 1 mole of gas does when it is heated isobarically by 1 K.
- The shape and structure of molecules are quite complex. But let's try to imagine them in the form of small balls. This will allow us to apply the laws of mechanics to the description of the process of impact of molecules on the vessel walls, in particular, Newton's second law.
- We assume that the gas molecules are at a sufficiently large distance from each other, so that the forces of interaction between them are negligible. If there are no interaction forces between the particles, the potential energy of interaction is equal to zero, respectively. Let's call a gas that meets these properties, perfect .
- It is known that gas molecules move at different speeds. However, let us average the velocities of the molecules and we will consider them the same.
- Let us assume that the impacts of molecules on the walls of the vessel are absolutely elastic (the molecules behave on impact like rubber balls, and not like a piece of plasticine). In this case, the velocities of the molecules change only in direction, but remain the same in magnitude. Then the change in the velocity of each molecule upon impact is –2υ.
Having introduced such simplifications, we calculate the pressure of the gas on the walls of the vessel.
|
|
The force acts on the wall from many molecules. It can be calculated as the product of the force acting from one molecule and the number of molecules moving in the vessel in the direction of this wall. Since space is three-dimensional and each dimension has two directions: positive and negative, we can assume that one sixth of all molecules (with a large number of them) move in the direction of one wall: N = N 0 / 6.
The force acting on the wall from one molecule is equal to the force acting on the molecule from the side of the wall. The force acting on the molecule from the side of the wall is equal to the product of the mass of one molecule and the acceleration that it receives when it hits the wall:
|
Acceleration, on the other hand, is a physical quantity determined by the ratio of the change in speed to the time during which this change occurred: a = Δυ / t.
The change in speed is equal to the double value of the speed of the molecule before the impact: Δυ = –2υ .
If a molecule behaves like a rubber ball, it is not difficult to imagine the process of impact: the molecule, upon impact, is deformed. The process of compression and decompression takes time. While the molecule acts on the wall of the vessel, a certain number of molecules still have time to hit the latter, located at distances not farther from it l = υt. (For example, relatively speaking, let the molecules have a speed of 100 m / s. The impact lasts 0.01 s. Then during this time the molecules will have time to reach the wall and contribute to the pressure of the molecules located at distances of 10, 50, 70 cm from it, but not more than 100 cm).
We will consider the volume of the vessel V = lS .
Substituting all the formulas into the original one, we get the equation:
|
where: is the mass of one molecule, is the average value of the square of the velocity of molecules, N is the number of molecules in the volume V .
Let us make some explanations about one of the quantities included in the resulting equation.
Since the movement of molecules is chaotic and there is no predominant movement of molecules in the vessel, their average velocity is zero. But it is clear that this does not apply to every single molecule.
To calculate the pressure of an ideal gas on the vessel wall, not the average value of the x-component of the molecular velocity is used, but the average value of the square of the velocity
To make the introduction of this quantity more understandable, consider a numerical example.
Let four molecules have velocities of 1, 2, 3, 4 arb. units
The square of the average velocity of molecules is:
|
The average value of the square of the speed is:
|
The average values of the projections of the square of the velocity on the x, y, z axes are related to the average value of the square of the velocity by the ratio.
Question 1
The main provisions of the ICT and their experimental substantiation.?
1. All substances are composed of molecules, i.e. have a discrete structure, the molecules are separated by gaps.
2. Molecules are in continuous random (chaotic) motion.
3. Between the molecules of the body there are forces of interaction.
Brownian motion?.
Brownian motion is the continuous random motion of particles suspended in a gas.
Forces of molecular interaction?.
Both attraction and repulsion act simultaneously between molecules. The nature of the interaction of molecules is electromagnetic.
Kinetic and potential energy of molecules?.
Atoms and molecules interact and, therefore, have a potential energy E p.
Potential energy is considered positive when molecules are repelled, negative when they are attracted.
Question 2
Dimensions and masses of molecules and atoms
Any substance consists of particles, therefore the amount of substance v (nu) is considered to be proportional to the number of particles, i.e. structural elements contained in the body.
The unit of quantity of a substance is the mole. A mole is the amount of a substance that contains as many structural elements of any substance as there are atoms in 12 g of C12 carbon. The ratio of the number of molecules of a substance to the amount of a substance is called the Avogadro constant:
N A =N/v(nu); N A \u003d 6.02 * 10 23 mol -1
The Avogadro constant shows how many atoms and molecules are contained in one mole of a substance. Molar mass - the mass of one mole of a substance, equal to the ratio of the mass of the substance to the amount of the substance:
Molar mass is expressed in kg/mol. Knowing the molar mass, you can calculate the mass of one molecule:
m 0 \u003d m / N \u003d m / v (nu) N A \u003d M / N A
The average mass of molecules is usually determined chemical methods, the Avogadro constant is determined with high accuracy by several physical methods. The masses of molecules and atoms are determined with a considerable degree of accuracy using a mass spectrograph.
The masses of molecules are very small. For example, the mass of a water molecule: m = 29.9 * 10 -27
The molar mass is related to the relative molecular mass Mg. Relative molecular mass- this is a value equal to the ratio of the mass of a molecule of a given substance to 1/12 of the mass of a carbon atom C12. If known chemical formula substance, then using the periodic table can be determined by its relative mass, which, when expressed in kilograms, gives the value of the molar mass of this substance.
Avogadro's number
Avogadro's number, Avogadro's constant is a physical constant numerically equal to the number of specified structural units(atoms, molecules, ions, electrons or any other particles) in 1 mole of a substance. Defined as the number of atoms in 12 grams (exactly) of the pure carbon-12 isotope. It is usually designated as N A, less often as L
N A = 6.022 140 78(18)×1023 mol −1 .
Number of moles
Mole (symbol: mol, international: mol) is a unit of measure for the amount of a substance. Corresponds to the amount of a substance that contains N A particles (molecules, atoms, ions, or any other identical structural particles). N A is Avogadro's constant, equal to the number of atoms in 12 grams of the carbon nuclide 12C. Thus, the number of particles in one mole of any substance is constant and equal to the Avogadro number N A .
Molecule speed
State of matter
Aggregate state - a state of matter characterized by certain quality properties: the ability or inability to maintain volume and shape, the presence or absence of long-range and short-range order, and others. A change in the state of aggregation may be accompanied by a jump-like change in free energy, entropy, density, and other basic physical properties.
There are three main states of aggregation: solid, liquid and gas. Sometimes it is not entirely correct to classify plasma as a state of aggregation. There are other states of aggregation, for example, liquid crystals or Bose-Einstein condensate.
Question 3
Ideal gas, gas pressure
An ideal gas is a gas in which there is no interaction force between molecules.
The pressure of a gas is due to the impacts of molecules. The force of pressure for 1 second on a unit surface is called gas pressure.
P – gas pressure [pa]
1 mmHg Art. =133 Pa
P 0 (ro) \u003d 101325 Pa
P= 1/3*m 0 *n*V 2- the basic equation of the MKT
n - concentration of molecules [m -3]
n=N/V- concentration of molecules
V 2 - root mean square speed
P= 2/3*n*E K basic equations
P= n*k*T MKT
E K - kinetic energy
E K = 3/2kT(kT- kote)
How does the pressure of an ideal gas change?
An ideal gas is a physical model of a gas. This model practically does not take into account the interaction of molecules with each other. It is used to describe the behavior of gases from a mathematical point of view. This model assumes the following gas properties:
- the size of the molecules is greater than the distance between the molecules;
- molecules are round balls;
- molecules repel each other and from the walls of the vessel only after the collision. The collisions are perfectly elastic;
- Molecules move according to Newton's laws.
There are several types of ideal gas:
- classical;
- quantum (considers an ideal gas under conditions of a decrease in temperature and an increase in the distance between molecules);
- in a gravitational field (considers changes in the properties of an ideal gas in a gravitational field).
The classical ideal gas will be considered below.
How to determine the pressure of an ideal gas?
The fundamental dependence of all ideal gases is expressed using the Mendeleev-Clapeyron equation.
PV=(m/M).RT [Formula 1]
- P is pressure. Unit of measure - Pa (Pascal)
- R=8.314 is the universal gas constant. Unit of measurement - (J / mol.K)
- T - temperature
- V - volume
- m is the mass of gas
- M is the molar mass of the gas. The unit of measurement is (g/mol).
P = nkT [Formula 2]
Formula 2 shows that the pressure of an ideal gas depends on the concentration of molecules and temperature. If we take into account the features of an ideal gas, then n will be determined by the formula:
n = mNа/MV [Formula 3]
- N is the number of molecules in the vessel
- N a - Avogadro's constant
Substituting formula 3 into formula 2, we get:
- PV = (m/M)Na kT [Formula 4]
- k*N a = R [Formula 5]
The constant R is a constant for one mole of gas in the Mendeleev-Clapeyron equation (remember: at constant pressure and temperature, 1 mole of different gases occupies the same volume).
Now we derive the pressure equation for an ideal gas
m/M = ν [Formula 6]
- where ν are the quantities of matter. Unit of measure - mol
We obtain the ideal gas pressure equation, the formula is given below:
P=νRT/V [Formula 7]
- where P is the pressure. Unit of measure - Pa (Pascal)
- R= 8.314 is the universal gas constant. Unit of measurement - (J / mol.K)
- T - temperature
- V is the volume.
How will the pressure of an ideal gas change?
Analyzing equation 7, we can see that the pressure of an ideal gas is proportional to the change in temperature and concentration.
In the state of an ideal gas, changes in all the parameters on which it depends are possible, and changes in some of them are also possible. Consider the most likely situations:
- isothermal process. This process is characterized by the fact that the temperature in it will be constant (T = const). If we substitute a constant temperature in equation 1, we will see that the value of the product P * V will also be constant.
- PV = const [Formula 8]
Equation 8 shows the relationship between the volume of a gas and its pressure at a constant temperature. This equation was experimentally discovered in the 17th century by the physicists Robert Boyle and Edme Mariotte. The equation was named after them the Boyle-Mariotte law.
- isochoric process. In this process, the volume, mass of the gas and its molar mass remain constant. V=const, m=const, M=const. Thus, we obtain the pressure of an ideal gas. The formula is shown below:
- P= P 0 AT [Formula 9]
- Where: P is the gas pressure at absolute temperature,
- P 0 - gas pressure at a temperature of 273 ° K (0 ° C),
- A is the pressure temperature coefficient. A \u003d (1 / 273.15) K -1
This relationship was discovered experimentally in the 19th century by the physicist Charles. Therefore, the equation bears the name of its creator - Charles' law.
An isochoric process can be observed if the gas is heated at a constant volume.
- isobaric process. For this process, pressure, gas mass and its molar mass will be constant. P = const, m = const, M = const. The isobaric process equation has the form:
- V/T = const or V = V 0 AT [Formula 10]
- where: V 0 is the volume of gas at 273° K (0° C);
- A \u003d (1 / 273.15) K -1.
In this formula, the coefficient A acts as a temperature coefficient for the volumetric expansion of the gas.
This relationship was discovered in the 19th century by the physicist Joseph Gay-Lussac. That is why this equality bears his name - Guy-Lussac's law.
If we take a glass flask connected to a tube, the opening of which will be closed by a liquid, and heat the structure, then it will be possible to observe an isobaric process.
It should be noted that air at room temperature has properties similar to an ideal gas.
Instruction
Find pressure ideal gas if there are values average speed, mass of one molecule and concentration according to the formula P=⅓nm0v2, where n is the concentration (in grams or moles per liter), m0 is the mass of one molecule.
Calculate pressure if you know the temperature gas and its concentration, using the formula P=nkT, where k is Boltzmann's constant(k=1.38 10-23 mol K-1), T is the temperature on the absolute Kelvin scale.
Find pressure from two equivalent versions of the Mendeleev-Claiperon equation depending on the known values: P=mRT/MV or P=νRT/V, where R is the universal gas constant (R=8.31 J/mol K), ν - in moles, V - volume gas in m3.
If the condition of the problem specifies the average of molecules gas and its concentration, find pressure using the formula P=⅔nEk, where Ek is the kinetic energy in J.
Find pressure from gas laws- isochoric (V=const) and isothermal (T=const) if given pressure in one of the states. With an isochoric process, the ratio of pressures in two states is equal to the ratio: P1/P2=T1/T2. In the second case, if the temperature remains constant value, product of pressure gas by its volume in the first state is equal to the same product in the second state: P1·V1=P2·V2. Express the unknown quantity.
When calculating the partial pressure of steam, if the temperature and air are given in the condition, express pressure from the formula φ / 100 \u003d P1 / P2, where φ / 100 - relative humidity, P1 - partial pressure water vapor, P2 - the maximum value of water vapor at a given temperature. When calculating, use the tables of maximum vapor pressure (maximum partial pressure) versus temperature in degrees Celsius.
Use an aneroid barometer or a mercury barometer for a more accurate value if you need to calculate gas pressure during an experiment or laboratory work. To measure the gas pressure in a vessel or cylinder, use a conventional or electronic pressure gauge.
Sources:
- Pressure and density of saturated water vapor depending on temperature - table
- gas pressure formula
Will the bucket hold up if you pour water into it? And if you pour a heavier liquid there? To answer this question, it is necessary to calculate pressure, which exerts a liquid on the walls of a vessel. This is very often necessary in production - for example, in the manufacture of tanks or tanks. It is especially important to calculate the strength of containers if we are talking about hazardous liquids.
You will need
- Vessel
- Liquid with known density
- Knowledge of Pascal's Law
- Hydrometer or pycnometer
- measuring beaker
- Correction table for weighing in air
- Ruler
Instruction
Sources:
- Calculation of liquid pressure on the bottom and walls of the vessel
Even with a little effort, you can create a significant pressure. All that is needed for this is to concentrate this effort on a small area. Conversely, if a significant force is evenly distributed over a large area, pressure turn out to be relatively small. To find out exactly how, you will have to carry out a calculation.

Instruction
If the task does not show the force, but the mass of the load, calculate the force using the following formula: F \u003d mg, where F is the force (N), m is the mass (kg), g is the free fall acceleration, equal to 9.80665 m / s².
If in the conditions, instead of the area, the geometric parameters of the area on which the pressure, first calculate the area of this area. For example, for a rectangle: S=ab, where S is the area (m²), a is the length (m), b is the width (m). For a circle: S=πR², where S is the area (m²), π is the number " pi", 3.1415926535 (dimensionless value), R - radius (m).
To find out pressure, divide the force by the area: P=F/S, where P is pressure(Pa), F - force (n), S - area (m²).
In the course of preparing accompanying documentation for goods intended for export, it may be necessary to express pressure in pounds per square inch (PSI - pounds per square inch). In this case, be guided by the following ratio: 1 PSI = 6894.75729 Pa.