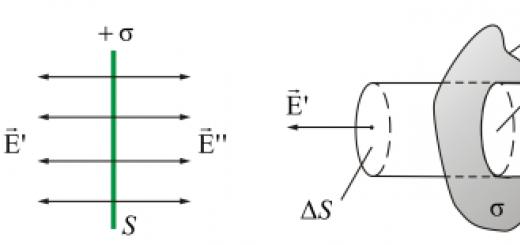
Chemical equilibrium is such a state of reversible chemical reaction
aA + b B= c C+ d D,
at which over time there is no change in the concentrations of the reactants in the reaction mixture. The state of chemical equilibrium is characterized constant chemical equilibrium :
where C i are the concentrations of components in equilibrium the perfect mixture.
The equilibrium constant can also be expressed in terms of equilibrium mole fractions X i components:
For reactions occurring in the gas phase, it is convenient to express the equilibrium constant in terms of the equilibrium partial pressures Pi components:
For ideal gases Pi = C i RT and Pi = X i P, where P is the total pressure, so KP, K C and K X are related by the following relation:
K P = K C (RT) c+d–a–b = K X P c+d–a–b. (9.4)
The equilibrium constant is related to rG o chemical reaction:
![]() (9.5)
(9.5)
![]() (9.6)
(9.6)
Change rG or r F in a chemical reaction at given (not necessarily equilibrium) partial pressures Pi or concentrations C i components can be calculated by the equation chemical reaction isotherms (van't Hoff isotherms):
 . (9.7)
. (9.7)
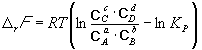 . (9.8)
. (9.8)
According to principle of Le Chatelier If an external force is exerted on a system in equilibrium, then the equilibrium will shift in such a way as to reduce the effect of the external force. Thus, an increase in pressure shifts the equilibrium in the direction of a decrease in the number of gas molecules. The addition of a reaction component to an equilibrium mixture shifts the equilibrium in the direction of decreasing the amount of this component. An increase (or decrease) in temperature shifts the equilibrium in the direction of a reaction proceeding with the absorption (release) of heat.
Quantitatively, the dependence of the equilibrium constant on temperature is described by the equation isobars of a chemical reaction (van't Hoff isobars)
![]() (9.9)
(9.9)
and isochores of a chemical reaction (van't Hoff isochores)
![]() . (9.10)
. (9.10)
Integration of equation (9.9) under the assumption that rH reaction does not depend on temperature (which is true in narrow temperature ranges), gives:
![]() (9.11)
(9.11)
![]() (9.12)
(9.12)
where C- integration constant. Thus, the dependence ln K P from 1 /T must be linear, and the slope of the straight line is - rH/R.
Integration within K 1 , K 2 , and T 1, T 2 gives:
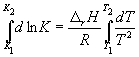 (9.13)
(9.13)
 (9.14)
(9.14)
Using this equation, knowing the equilibrium constants at two different temperatures, we can calculate rH reactions. Accordingly, knowing rH reaction and the equilibrium constant at one temperature, you can calculate the equilibrium constant at another temperature.
EXAMPLES
CO (g) + 2H 2 (g) \u003d CH 3 OH (g)
at 500K. f G o for CO(g) and CH 3 OH(g) at 500 K are –155.41 kJ. mol –1 and –134.20 kJ. mol –1, respectively.
Decision. Go reactions:
r G o= f G o(CH 3 OH) - f G o(CO) = –134.20 – (–155.41) = 21.21 kJ. mol -1 .
![]() = 6.09 10 –3 .
= 6.09 10 –3 .
Example 9-2. Reaction equilibrium constant
is equal to K P = 1.64 10 –4 at 400 o C. What total pressure must be applied to an equimolar mixture of N 2 and H 2 to convert 10% N 2 into NH 3 ? The gases are assumed to be ideal.
Decision. Let mol N 2 react. Then
| N 2 (g) | + | 3H 2 (g) | = | 2NH 3 (g) | |
| Initial quantity | 1 | 1 | |||
| Equilibrium amount | 1– | 1–3 | 2 (Total: 2–2) | ||
| Equilibrium mole fraction: |
Hence, K X=  and K P = K X . P –2
=
and K P = K X . P –2
=  .
.
Substituting = 0.1 into the resulting formula, we have
1.64 10 –4 = , where P= 51.2 atm.
, where P= 51.2 atm.
Example 9-3. Reaction equilibrium constant
CO (g) + 2H 2 (g) \u003d CH 3 OH (g)
at 500 K is K P = 6.0910–3. The reaction mixture consisting of 1 mol of CO, 2 mol of H 2 and 1 mol of an inert gas (N 2) is heated to 500 K and a total pressure of 100 atm. Calculate the composition of the equilibrium mixture.
Decision. Let a mole of CO react. Then
| CO(g) | + | 2H 2 (g) | = | CH 3 OH (g) | |
| Initial amount: | 1 | 2 | 0 | ||
| Equilibrium amount: | 1– | 2–2 | |||
| Total in the equilibrium mixture: | 3–2 mol components + 1 mol N 2 \u003d 4–2 mol | ||||
| Equilibrium mole fraction | |||||
Hence, K X=  and K P = K X . P-2 =
and K P = K X . P-2 = ![]() .
.
Thus, 6.09 10 –3 = ![]() .
.
Solving this equation, we get = 0.732. Accordingly, the molar fractions of substances in the equilibrium mixture are: = 0.288, = 0.106, = 0.212, and = 0.394.
Example 9-4. For reaction
N 2 (g) + 3H 2 (g) \u003d 2NH 3 (g)
at 298 K K P = 6.0 10 5 , and f H o(NH 3) \u003d -46.1 kJ. mol -1 . Estimate the value of the equilibrium constant at 500 K.
Decision. The standard molar enthalpy of reaction is
r H o= 2f H o(NH 3) \u003d -92.2 kJ. mol -1 .
According to equation (9.14), 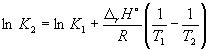 =
=
Ln (6.0 10 5) + ![]() = –1.73, whence K 2 =
0.18.
= –1.73, whence K 2 =
0.18.
Note that the equilibrium constant of an exothermic reaction decreases with increasing temperature, which corresponds to Le Chatelier's principle.
TASKS
- At 1273 K and a total pressure of 30 atm in an equilibrium mixture
- At 2000 o C and a total pressure of 1 atm, 2% of water is dissociated into hydrogen and oxygen. Calculate the equilibrium constant of the reaction
- Reaction equilibrium constant
- Reaction equilibrium constant
- A 3-L vessel containing 1.7910–2 mol I 2 was heated to 973 K. The pressure in the vessel at equilibrium turned out to be 0.49 atm. Assuming ideal gases, calculate the equilibrium constant at 973 K for the reaction
- For reaction
- For reaction
- A 1-liter vessel containing 0.341 mol PCl 5 and 0.233 mol N 2 was heated to 250 o C. The total pressure in the vessel at equilibrium was 29.33 atm. Considering all gases to be ideal, calculate the equilibrium constant at 250 o C for the reaction taking place in the vessel
- Reaction equilibrium constant
- At 25°C f G o(NH 3) = –16.5 kJ. mol -1 . Calculate rG reactions of formation of NH 3 at partial pressures of N 2 , H 2 and NH 3 equal to 3 atm, 1 atm and 4 atm, respectively. In which direction will the reaction proceed spontaneously under these conditions?
- exothermic reaction
- The equilibrium constant of the gas phase isomerization reaction of borneol (C10H17OH) to isoborneol is 0.106 at 503 K. A mixture of 7.5 g of borneol and 14.0 g of isoborneol was placed in a 5 L vessel and kept at 503 K until equilibrium was reached. Calculate the mole fractions and masses of borneol and isoborneol in an equilibrium mixture.
- Equilibrium in reaction
- Calculate the total pressure that must be applied to a mixture of 3 parts H 2 and 1 part N 2 to obtain an equilibrium mixture containing 10% NH 3 by volume at 400 o C. The equilibrium constant for the reaction
- At 250 o C and a total pressure of 1 atm, PCl 5 is dissociated by 80% according to the reaction
- At 2000 o C for the reaction
- Calculate the standard enthalpy of the reaction for which the equilibrium constant is
a) increases by 2 times, b) decreases by 2 times when the temperature changes from 298 K to 308 K. - The dependence of the equilibrium constant of the reaction 2C 3 H 6 (g) \u003d C 2 H 4 (g) + C 4 H 8 (g) on temperature between 300 K and 600 K is described by the equation
CO 2 (g) + C (tv) \u003d 2CO (g)
contains 17% (by volume) CO 2 . What percentage of CO 2 will be contained in the gas at a total pressure of 20 atm? At what pressure will the gas contain 25% CO 2 ?
H 2 O (g) \u003d H 2 (g) + 1 / 2O 2 (g) under these conditions.
CO (g) + H 2 O (g) \u003d CO 2 (g) + H 2 (g)
at 500 o C is Kp= 5.5. A mixture of 1 mol CO and 5 mol H 2 O was heated to this temperature. Calculate the mole fraction of H 2 O in the equilibrium mixture.
N 2 O 4 (g) \u003d 2NO 2 (g)
at 25 o C is Kp= 0.143. Calculate the pressure that will be established in a 1 liter vessel in which 1 g of N 2 O 4 is placed at this temperature.
I 2 (g) = 2I (g).
at 250°C rG o \u003d -2508 J. mol -1. At what total pressure will the degree of conversion of PCl 5 to PCl 3 and Cl 2 at 250 o C be 30%?
2HI (g) \u003d H 2 (g) + I 2 (g)
equilibrium constant K P = 1.83 10 –2 at 698.6 K. How many grams of HI are formed when 10 g of I 2 and 0.2 g of H 2 are heated to this temperature in a three-liter vessel? What are the partial pressures of H 2 , I 2 and HI?
PCl 5 (g) = PCl 3 (g) + Cl 2 (g)
CO (g) + 2H 2 (g) \u003d CH 3 OH (g)
at 500 K is K P = 6.0910–3. Calculate the total pressure required to produce methanol with 90% yield if CO and H 2 are taken in a 1:2 ratio.
CO (g) + 2H 2 (g) \u003d CH 3 OH (g)
is in equilibrium at 500 K and 10 bar. If the gases are ideal, how will the following factors affect the yield of methanol: a) increasing T; b) promotion P; c) adding an inert gas at V= const; d) addition of an inert gas at P= const; e) adding H 2 at P= const?
2NOCl (g) \u003d 2NO (g) + Cl 2 (g)
set at 227 o C and a total pressure of 1.0 bar, when the partial pressure of NOCl is equal to 0.64 bar (initially only NOCl was present). Calculate r G o for a reaction. At what total pressure will the partial pressure of Cl 2 be 0.10 bar?
N 2 (g) + 3H 2 (g) \u003d 2NH 3 (g)
at 400 o C is K = 1.60 10 –4 .
PCl 5 (g) = PCl 3 (g) + Cl 2 (g).
What will be the degree of dissociation of PCl 5 if N 2 is added to the system so that the partial pressure of nitrogen is 0.9 atm? The total pressure is maintained at 1 atm.
N 2 (g) + O 2 (g) \u003d 2NO (g)
Kp = 2.510–3. An equilibrium mixture of N 2 , O 2 , NO and an inert gas at a total pressure of 1 bar contains 80% (by volume) N 2 and 16% O 2 . What percentage by volume is NO? What is the partial pressure of an inert gas?
ln K = –1.04 –1088 /T +1.51 10 5 /T 2 .
In 1885, the French physicist and chemist Le Chatelier was deduced, and in 1887 by the German physicist Braun, the law of chemical equilibrium and the chemical equilibrium constant were substantiated, and their dependence on the influence of various external factors was studied.
The essence of chemical equilibrium
Equilibrium is a state that means things are always moving. Products are decomposed into reagents, and reagents are combined into products. Things move, but concentrations remain the same. The reaction is written with a double arrow instead of an equals sign to show that it is reversible.

Classic patterns
Back in the last century, chemists discovered certain patterns that provide for the possibility of changing the direction of the reaction in the same container. Knowledge of how chemical reactions proceed is incredibly important, both for laboratory research and industrial production. Wherein great importance has the ability to control all of these phenomena. It is human nature to intervene in many natural processes, especially reversible ones, in order to later use them for their own benefit. From knowledge of chemical reactions will be more useful if you are fluent in the levers of controlling them.
The law of mass action in chemistry is used by chemists to correctly calculate the rates of reactions. It gives a clear idea that none will be completed if it takes place in a closed system. The molecules of the resulting substances are in constant and random motion, and a reverse reaction may soon occur, in which the molecules of the starting material will be restored.
In industry, open systems are most often used. Vessels, apparatus and other containers where chemical reactions take place remain unlocked. This is necessary so that during these processes it is possible to extract the desired product and get rid of useless reaction products. For example, coal is burned in open furnaces, cement is produced in open furnaces, blast furnaces operate with a constant supply of air, and ammonia is synthesized by continuously removing ammonia itself.

Reversible and irreversible chemical reactions
Based on the name, one can give the appropriate definitions: irreversible reactions are those that are brought to an end, do not change their direction and proceed along a given trajectory, regardless of pressure drops and temperature fluctuations. Their distinguishing feature is that some products may leave the reaction sphere. Thus, for example, it is possible to obtain gas (CaCO 3 \u003d CaO + CO 2), a precipitate (Cu (NO 3) 2 + H 2 S \u003d CuS + 2HNO 3) or others will also be considered irreversible if during the process it is released a large number of thermal energy, for example: 4P + 5O 2 \u003d 2P 2 O 5 + Q.
Almost all reactions that occur in nature are reversible. Regardless of such external conditions as pressure and temperature, almost all processes can proceed simultaneously in different directions. As the law of mass action in chemistry says, the amount of heat absorbed will be equal to the amount released, which means that if one reaction was exothermic, then the second (reverse) will be endothermic.
Chemical equilibrium: chemical equilibrium constant
Reactions are the "verbs" of chemistry - the activities that chemists study. Many reactions go to completion and then stop, meaning that the reactants are completely converted to products without being able to return to their original state. In some cases, the reaction is indeed irreversible, for example, when combustion changes both physical and chemical. However, there are many other circumstances in which it is not only possible, but also continuous, since the products of the first reaction become reactants in the second.
The dynamic state in which the concentrations of reactants and products remain constant is called equilibrium. It is possible to predict the behavior of substances with the help of certain laws that are applied in industries seeking to reduce the cost of producing specific chemicals. The concept of chemical equilibrium is also useful in understanding processes that maintain or potentially threaten human health. The chemical equilibrium constant is the value of a reaction factor that depends on ionic strength and temperature and is independent of the concentrations of reactants and products in solution.

Calculation of the equilibrium constant
This value is dimensionless, that is, it does not have a certain number of units. Although the calculation is usually written for two reactants and two products, it works for any number of reaction participants. The calculation and interpretation of the equilibrium constant depends on whether the chemical reaction is associated with a homogeneous or heterogeneous equilibrium. This means that all reacting components can be pure liquids or gases. For reactions that reach heterogeneous equilibrium, as a rule, not one phase is present, but at least two. For example, liquids and gases or and liquids.

The value of the equilibrium constant
For any given temperature, there is only one value for the equilibrium constant, which only changes if the temperature at which the reaction occurs changes in one direction or another. Some predictions about a chemical reaction can be made based on whether the equilibrium constant is large or small. If the value is very large, then the equilibrium favors the reaction to the right and more products are obtained than there were reactants. The reaction in this case can be called "total" or "quantitative".
If the value of the equilibrium constant is small, then it favors the reaction to the left, where the amount of reactants was greater than the number of products formed. If this value tends to zero, we can assume that the reaction does not occur. If the values of the equilibrium constant for the direct and reverse reactions are almost the same, then the amount of reactants and products will also be almost the same. This type of reaction is considered to be reversible.

Consider a specific reversible reaction
Let's take two chemical element, like iodine and hydrogen, which, when mixed, give a new substance - hydrogen iodide.
For v 1 we take the rate of the direct reaction, for v 2 - the rate of the reverse reaction, k - the equilibrium constant. Using the law of mass action, we obtain the following expression:
v 1 \u003d k 1 * c (H 2) * c (I 2),
v 2 = k 2 * c 2 (HI).
When mixing iodine (I 2) and hydrogen (H 2) molecules, their interaction begins. At the initial stage, the concentration of these elements is maximum, but by the end of the reaction, the concentration of a new compound, hydrogen iodide (HI), will be maximum. Accordingly, the reaction rates will also be different. At the very beginning, they will be maximum. Over time, there comes a moment when these values are equal, and this is the state called chemical equilibrium.
The expression of the chemical equilibrium constant, as a rule, is denoted using square brackets: , , . Since at equilibrium the speeds are equal, then:
k 1 \u003d k 2 2,
so we get the equation of the chemical equilibrium constant:
k 1 /k 2 = 2 / = K.

Le Chatelier-Brown principle
There is the following regularity: if a certain effect is made on a system that is in equilibrium (change the conditions of chemical equilibrium by changing temperature or pressure, for example), then the balance will shift in order to partially counteract the effect of the change. In addition to chemistry, this principle also applies in several different forms to the fields of pharmacology and economics.

Chemical equilibrium constant and ways of its expression
The equilibrium expression can be expressed in terms of the concentration of products and reactants. Only chemical substances in the aqueous and gaseous phases are included in the equilibrium formula, since the concentrations of liquids and solids do not change. What factors affect chemical equilibrium? If it involves a pure liquid or solid, it is considered that it has K = 1, and, accordingly, ceases to be taken into account, with the exception of highly concentrated solutions. For example, pure water has an activity of 1.
Another example is solid carbon, which can be formed by the reaction of two molecules of carbon monoxide to form carbon dioxide and carbon. Factors that can affect the balance include the addition of a reactant or product (changes in concentration affect the balance). The addition of a reagent can bring equilibrium to the right in chemical equation where more product shapes appear. The addition of product can bring equilibrium to the left as more reactant forms become available.

Equilibrium occurs when a reaction proceeding in both directions has a constant ratio of products and reactants. In general, the chemical equilibrium is static, since the quantitative ratio of products and reactants is constant. However, a closer look reveals that equilibrium is actually a very dynamic process, as the reaction moves in both directions at the same rate.
Dynamic equilibrium is an example of a steady state function. For a system at steady state, the currently observed behavior continues into the future. Therefore, once the reaction reaches equilibrium, the ratio of product to reactant concentrations will remain the same even though the reaction continues.

How easy is it to talk about complex things?
Concepts such as chemical equilibrium and chemical equilibrium constant are quite difficult to understand. Let's take an example from life. Have you ever been stuck on a bridge between two cities and noticed that the traffic in the other direction is smooth and measured while you are hopelessly stuck in traffic? This is not good.
What if the cars were measured and at the same speed moving on both sides? Would the number of cars in both cities remain constant? When the speed of entry and exit to both cities is the same, and the number of cars in each city is stable over time, this means that the whole process is in dynamic equilibrium.
EXAMPLE
Calculate the chemical equilibrium constant for a reversible homogeneous reaction, CO + H 2 O \u003d CO 2 + H 2, based on the fact that the equilibrium of the concentration of substances:
[CO] p = 0.045 mol/l,
[H 2 O] p \u003d 0.064 mol / l,
[CO 2 ] p \u003d 0.18 mol / l.
Given:
[CO] p = 0.045 mol/l
[H 2 O] p \u003d 0.064 mol / l
[CO 2] p \u003d 0.18 mol / l
Decision:
The molar ratio of the reaction products is 1:1, therefore
[CO 2] p \u003d [H 2] p \u003d 0.18 mol / l.
Based on expression (2.1), we calculate the value of the chemical equilibrium constant:
K x.r. = [CO 2] p [H 2] p / [CO 2] p [H 2 O] p \u003d 0.18 0.18 / 0.045 0.064
Answer: 11,25.
2. Calculation of equilibrium concentrations from the initial concentrations of reactants and vice versa
EXAMPLE 1.
The reversible gas reaction proceeds according to the equation:
CO + CI 2 \u003d COCI 2.
Initial concentrations of reactants:
[CO] 0 \u003d 0.03 mol / l;
0 \u003d 0.02 mol / l.
After the onset of equilibrium, the concentration of carbon monoxide became:
[CO] p = 0.021 mol/L.
Calculate the equilibrium concentrations of the remaining substances and the value of the chemical equilibrium constant.
Given:
[CO] 0 \u003d 0.03 mol / l
[С1 2 ] 0 = 0.02 mol/l
[CO] p = 0.021 mol/l
P , p , K x . p-?
Decision:
By the time of equilibrium, the change in CO concentration was:
∆[CO] \u003d [CO] 0 - [CO] p \u003d 0.03 - 0.021 \u003d 0.009 mol / l.
Since the molar ratio of the substances involved in the reaction is 1:1:1, the change in the concentration of all substances is the same:
[C1 2] p \u003d [C1 2] 0 - ∆ [C1 2] \u003d 0.02 - 0.009 \u003d 0.011 mol / l,
[COS1 2 ] p = 0.009 mol/l,
K x p \u003d [COS1 2] P / [CO] P [C1 2] p \u003d 0.009 / 0.021 0.011 \u003d 39.
The results of the calculations will be entered in the table, where the signs "+" and "-" mean, respectively, an increase or decrease in the concentration of a substance.
Answer:[C1 2 ] p = 0.011 mol/l; [COS1 2 ] p = 0.009 mol/l; K xp = 39.
EXAMPLE 2.
The equilibrium concentrations of substances involved in the reversible reaction 2NO + O 2 \u003d 2NO 2 are as follows (mol / l):
P = 0.056;
[O 2 ] = 0.028;
Given:
P = 0.056 mol/l
P = 0.028 mol/l
P = 0.044 mol/l
0 , [О 2 ] 0 – ?
Decision:
The initial concentration of nitric oxide (IV) was 0 = 0, and its change by the moment of equilibrium is ∆ = 0.044 mol/l.
The molar ratio of NO and NO 2 in the reaction is 2:2 (1:1), therefore, the initial concentration of NO will be:
0 \u003d p + 0.044 \u003d 0.056 + 0.044 \u003d 0.1 mol / l.
The molar ratio of O 2 and NO 2 is 1:2, hence the initial concentration of O 2 will be:
[O 2] 0 \u003d [O 2] p + 0.044 / 2 \u003d 0.028 + 0.022 \u003d 0.05 mol / l.
We write the results of calculations in a table
Answer: 0 = 0.1 mol/l; [O 2] 0 \u003d 0.05 mol / l.
EXAMPLE 3.
The ammonia synthesis reaction proceeds according to the equation ZH 2 + N 2 = 2NH 3. Initial concentrations of initial substances are equal (mol/l): hydrogen – 0.05; nitrogen - 0.04: the reaction rate constant is 0.3. Calculate: a) the initial rate of the reaction; b) the reaction rate when the ammonia concentration became equal to 0.02 mol/l.
Given:
a) [H 2] 0 \u003d 0.05 mol / l
0 = 0.04 mol/l
b) = 0.02 mol/l
Decision:
a) In accordance with the law of mass action, we find the initial reaction rate:
υ 0 \u003d k 0 3 0 \u003d 3 10 -1 3 \u003d 1.5 10 -6 mol / l s.
b) Based on the reaction equation, the molar ratio of hydrogen and ammonia is 3:2. An increase in ammonia concentration by 0.02 mol/l causes a decrease in hydrogen concentration by 0.03 mol/l (0.02 - 3/2 = 0.03).
Thus, by the time when the ammonia concentration increased by 0.02 mol/l, the hydrogen concentration decreased to 0.02 mol/l (0.05 - 0.03 = 0.02). The molar ratio of nitrogen and ammonia is 1:2. The nitrogen concentration will decrease by 0.01 mol (0.02 - 1/2 = = 0.01) and become equal to 0.03 mol / l (0.04 - 0.01 = 0.03). The reaction rate will also decrease as the concentration of the reactants decreases:
υ \u003d k 3 \u003d 3 10 -1 3 \u003d 7.2 10 -8 mol / l s.
Answer: a) 1.5 10–6 mol/l s; b) 7.2 10–8 mol/l s.
EXAMPLE 4
The reaction proceeds according to the equation 2NO + O 2 \u003d 2NO 2, some time after the start of the reaction, the concentrations of all substances participating in the reaction became: \u003d 0.04 mol / l; [O 2 ] = 0.01 mol/l; = 0.02 mol/l. Calculate the initial concentrations of starting materials and the initial reaction rate if the reaction rate constant k = 1.
Given:
0.04 mol/l
[O 2] \u003d 0.01 mol / l
0.02 mol/l
0 , 0 , x 0 – ?
Decision:
According to the reaction equation, the molar ratio of NO and NO 2 is 2:2 (1:1).
An increase in the concentration of the reaction product NO 2 to 0.02 mol/l caused a decrease in the concentration of NO by 0.02 mol. Therefore, the initial concentration of nitric oxide (II) was:
0 \u003d +0.02 \u003d 0.04 + 0.02 \u003d 0.06 mol / l.
The molar ratio of O 2 and NO 2 is 1:2, therefore, an increase in the concentration of NO 2 to 0.02 mol caused a decrease in the oxygen concentration by 0.01 mol (0.02 1/2 \u003d 0.01). As a result, the initial oxygen concentration was:
[O 2] 0 \u003d [O 2] + 0.01 \u003d 0.01 + 0.01 \u003d 0.02 mol / l.
Initial reaction rate
υ 0 \u003d k 0 2 0 \u003d 1 2 \u003d 7.2 10 -5 mol / l s.
Answer: 0 = 0.06 mol/l; [O 2] 0 \u003d 0.02 mol / l;
x 0 \u003d 7.2 10 -5 mol / l s.
Most chemical reactions are reversible, i.e. flow simultaneously in opposite directions. In cases where the forward and reverse reactions proceed at the same rate, chemical equilibrium occurs. For example, in a reversible homogeneous reaction: H 2 (g) + I 2 (g) ↔ 2HI (g), the ratio of the rates of direct and reverse reactions according to the law of mass action depends on the ratio of the concentrations of the reactants, namely: the rate of the direct reaction: υ 1 = k 1 [Н 2 ]. The rate of the reverse reaction: υ 2 \u003d k 2 2.
If H 2 and I 2 are the initial substances, then at the first moment the rate of the forward reaction is determined by their initial concentrations, and the rate of the reverse reaction is zero. As H 2 and I 2 are consumed and HI is formed, the rate of the forward reaction decreases and the rate of the reverse reaction increases. After some time, both velocities are equalized, and chemical equilibrium is established in the system, i.e. the number of formed and consumed HI molecules per unit time becomes the same.
Since at chemical equilibrium the rates of direct and reverse reactions are equal to V 1 \u003d V 2, then k 1 \u003d k 2 2.
Since k 1 and k 2 are constant at a given temperature, their ratio will be constant. Denoting it by K, we get:

K - is called the constant of chemical equilibrium, and the above equation is called the law of mass action (Guldberg - Vaale).
AT general case for a reaction of the form аА+bB+…↔dD+eE+… the equilibrium constant is equal to ![]() . For interaction between gaseous substances often use the expression in which the reactants are represented by equilibrium partial pressures p. For the mentioned reaction
. For interaction between gaseous substances often use the expression in which the reactants are represented by equilibrium partial pressures p. For the mentioned reaction  .
.
The state of equilibrium characterizes the limit to which, under given conditions, the reaction proceeds spontaneously (∆G<0). Если в системе наступило химическое равновесие, то дальнейшее изменение изобарного потенциала происходить не будет, т.е. ∆G=0.
The ratio between the equilibrium concentrations does not depend on which substances are taken as starting materials (for example, H 2 and I 2 or HI), i.e. equilibrium can be approached from both sides.
The chemical equilibrium constant depends on the nature of the reactants and on the temperature; the equilibrium constant does not depend on pressure (if it is too high) and on the concentration of reagents.
Influence on the equilibrium constant of temperature, enthalpy and entropy factors. The equilibrium constant is related to the change in the standard isobaric-isothermal potential of a chemical reaction ∆G o by a simple equation ∆G o =-RT ln K.
It shows that large negative values of ∆G o (∆G o<<0) отвечают большие значения К, т.е. в равновесной смеси преобладают продукты взаимодействия. Если же ∆G o характеризуется большими положительными значениями (∆G o >>0), then the initial substances predominate in the equilibrium mixture. This equation allows us to calculate K from the value of ∆G o and then the equilibrium concentrations (partial pressures) of the reagents. If we take into account that ∆G o =∆Н o -Т∆S o , then after some transformation we get ![]() . It can be seen from this equation that the equilibrium constant is very sensitive to changes in temperature. The influence of the nature of the reagents on the equilibrium constant determines its dependence on the enthalpy and entropy factors.
. It can be seen from this equation that the equilibrium constant is very sensitive to changes in temperature. The influence of the nature of the reagents on the equilibrium constant determines its dependence on the enthalpy and entropy factors.
Le Chatelier's principle
The state of chemical equilibrium is maintained under these constant conditions at any time. When the conditions change, the state of equilibrium is disturbed, since in this case the rates of opposite processes change to different degrees. However, after some time, the system again comes to a state of equilibrium, but already corresponding to the new changed conditions.
The shift of equilibrium depending on changes in conditions is generally determined by the Le Chatelier principle (or the principle of moving equilibrium): if a system in equilibrium is influenced from outside by changing any of the conditions that determine the equilibrium position, then it is shifted in the direction of the process, the course of which weakens the effect of the effect produced.
Thus, an increase in temperature causes a shift in equilibrium in the direction of that of the processes, the course of which is accompanied by the absorption of heat, and a decrease in temperature acts in the opposite direction. Similarly, an increase in pressure shifts the equilibrium in the direction of a process accompanied by a decrease in volume, and a decrease in pressure acts in the opposite direction. For example, in the equilibrium system 3H 2 + N 2 2H 3 N, ∆H o = -46.2 kJ, an increase in temperature enhances the decomposition of H 3 N into hydrogen and nitrogen, since this process is endothermic. An increase in pressure shifts the equilibrium towards the formation of H 3 N, because the volume decreases.
If a certain amount of any of the substances participating in the reaction is added to a system that is in equilibrium (or vice versa, removed from the system), then the rates of the forward and reverse reactions change, but gradually become equal again. In other words, the system again comes to a state of chemical equilibrium. In this new state, the equilibrium concentrations of all substances present in the system will differ from the initial equilibrium concentrations, but the ratio between them will remain the same. Thus, in a system in equilibrium, it is impossible to change the concentration of one of the substances without causing a change in the concentrations of all the others.
In accordance with the Le Chatelier principle, the introduction of additional amounts of a reagent into the equilibrium system causes a shift in the equilibrium in the direction in which the concentration of this substance decreases and, accordingly, the concentration of the products of its interaction increases.
The study of chemical equilibrium is of great importance both for theoretical research and for solving practical problems. By determining the equilibrium position for various temperatures and pressures, one can choose the most favorable conditions for conducting a chemical process. In the final choice of process conditions, their influence on the process rate is also taken into account.
Example 1 Calculation of the equilibrium constant of the reaction from the equilibrium concentrations of the reactants.
Calculate the equilibrium constant of the reaction A + B 2C, if the equilibrium concentrations [A] = 0.3 mol ∙ l -1; [B]=1.1 mol∙l -1; [C] \u003d 2.1 mol ∙ l -1.
Decision. The expression for the equilibrium constant for this reaction is: . Let us substitute here the equilibrium concentrations indicated in the condition of the problem: =5.79.
Example 2. Calculation of equilibrium concentrations of reactants. The reaction proceeds according to the equation A + 2B C.
Determine the equilibrium concentrations of the reactants if the initial concentrations of substances A and B are respectively 0.5 and 0.7 mol∙l -1, and the equilibrium constant of the reaction K p =50.
Decision. For each mole of substances A and B, 2 moles of substance C are formed. If the decrease in the concentration of substances A and B is denoted by X mol, then the increase in the concentration of the substance will be equal to 2X mol. The equilibrium concentrations of the reactants will be:
C A \u003d (o.5-x) mol ∙ l -1; C B \u003d (0.7-x) mol ∙ l -1; C C \u003d 2x mol ∙ l -1
x 1 \u003d 0.86; x 2 \u003d 0.44
According to the condition of the problem, the value x 2 is valid. Hence, the equilibrium concentrations of the reactants are:
C A \u003d 0.5-0.44 \u003d 0.06 mol ∙ l -1; C B \u003d 0.7-0.44 \u003d 0.26 mol ∙ l -1; C C \u003d 0.44 ∙ 2 \u003d 0.88 mol ∙ l -1.
Example 3 Determination of the change in the Gibbs energy ∆G o of the reaction by the value of the equilibrium constant K p. Calculate the Gibbs energy and determine the possibility of the reaction CO+Cl 2 =COCl 2 at 700K, if the equilibrium constant is Kp=1.0685∙10 -4. The partial pressure of all reacting substances is the same and equal to 101325 Pa.
Decision.∆G 700 =2.303∙RT ![]() .
.
For this process:
Since ∆Go<0, то реакция СО+Cl 2 COCl 2 при 700К возможна.
Example 4. Shift in chemical equilibrium. In which direction will the equilibrium shift in the N 2 + 3H 2 2NH 3 -22 kcal system:
a) with an increase in the concentration of N 2;
b) with an increase in the concentration of H 2;
c) when the temperature rises;
d) when the pressure decreases?
Decision. An increase in the concentration of substances on the left side of the reaction equation, according to the Le Chatelier rule, should cause a process that tends to weaken the effect, lead to a decrease in concentrations, i.e. the equilibrium will shift to the right (cases a and b).
The ammonia synthesis reaction is exothermic. An increase in temperature causes a shift in equilibrium to the left - towards an endothermic reaction that weakens the impact (case c).
A decrease in pressure (case d) will favor the reaction leading to an increase in the volume of the system, i.e. towards the formation of N 2 and H 2 .
Example 5 How many times will the rate of forward and reverse reactions in the system 2SO 2 (g) + O 2 (g) 2SO 3 (r) change if the volume of the gas mixture decreases three times? In which direction will the equilibrium of the system shift?
Decision. Let us denote the concentrations of reacting substances: = a, =b,=with. According to the law of mass action, the rates of the forward and reverse reactions before a change in volume are
v pr \u003d Ka 2 b, v arr \u003d K 1 s 2
After reducing the volume of a homogeneous system by a factor of three, the concentration of each of the reactants will increase by a factor of three: 3a,[O 2] = 3b; = 3s. At new concentrations of the rate v "np of the direct and reverse reactions:
v" np = K(3a) 2 (3b) = 27 Ka 2 b; v o 6 p = K 1 (3c) 2 = 9K 1 c 2 .
![]() ;
; ![]()
Consequently, the rate of the forward reaction increased 27 times, and the reverse - only nine times. The equilibrium of the system has shifted towards the formation of SO 3 .
Example 6 Calculate how many times the rate of the reaction proceeding in the gas phase will increase with an increase in temperature from 30 to 70 0 C, if the temperature coefficient of the reaction is 2.
Decision. The dependence of the rate of a chemical reaction on temperature is determined by the Van't Hoff empirical rule according to the formula
Therefore, the reaction rate at 70°C is 16 times greater than the reaction rate at 30°C.
Example 7 The equilibrium constant of a homogeneous system
CO (g) + H 2 O (g) CO 2 (g) + H 2 (g) at 850 ° C is 1. Calculate the concentrations of all substances at equilibrium if the initial concentrations are: [CO] ISC = 3 mol / l, [H 2 O] ISH \u003d 2 mol / l.
Decision. At equilibrium, the rates of the forward and reverse reactions are equal, and the ratio of the constants of these rates is constant and is called the equilibrium constant of the given system:
V np= K 1[CO][H 2 O]; V o b p = To 2 [CO 2 ][H 2 ];
![]()
In the condition of the problem, the initial concentrations are given, while in the expression K r includes only the equilibrium concentrations of all substances in the system. Let us assume that by the moment of equilibrium the concentration [СО 2 ] Р = X mol/l. According to the equation of the system, the number of moles of hydrogen formed in this case will also be X mol/l. The same number of prayers (X mol / l) CO and H 2 O are consumed for the formation of X moles of CO 2 and H 2. Therefore, the equilibrium concentrations of all four substances (mol / l):
[CO 2] P \u003d [H 2] p \u003d X;[CO] P = (3 – x); P =(2-x).
Knowing the equilibrium constant, we find the value X, and then the initial concentrations of all substances:
![]() ; x 2 \u003d 6-2x-3x + x 2; 5x \u003d 6, l \u003d 1.2 mol / l.
; x 2 \u003d 6-2x-3x + x 2; 5x \u003d 6, l \u003d 1.2 mol / l.
All chemical reactions can be divided into reversible and irreversible. Reversible reactions are those that, at a certain temperature, proceed at a noticeable rate in two opposite directions - forward and reverse. Reversible reactions do not proceed to the end, none of the reactants is completely consumed. An example is the reaction
In a certain temperature range, this reaction is reversible. Sign " » is the sign of reversibility.
Irreversible reactions are those reactions that proceed only in one direction to the end, i.e. until the complete consumption of one of the reactants. An example of an irreversible reaction is the decomposition of potassium chlorate:
The formation of potassium chlorate from potassium chloride and oxygen is impossible under normal conditions.
state of chemical equilibrium. Chemical equilibrium constant
Let us write the equation of some reversible reaction in general form:
By the time the reaction started, the concentrations of starting substances A and B were maximum. During the reaction, they are consumed and their concentration decreases. In this case, in accordance with the law of mass action, the rate of the direct reaction
will decrease. (Hereinafter, the arrow at the top indicates the direction of the process.) At the initial moment, the concentrations of the reaction products D and E were equal to zero. During the reaction, they increase, the rate of the reverse reaction increases from zero according to the equation:
On fig. 4.5 shows the change in forward and reverse speeds
reactions over time. After time t, these speeds are equal - - " 
Rice. 4.5. Change in the rate of direct (1) and reverse (2) reactions in time: - in the absence of a catalyst: .......... - in the presence of a catalyst
This state is called chemical equilibrium. Chemical equilibrium is the most stable, limiting state of spontaneous processes. It can continue indefinitely if external conditions are not changed. In isolated systems in a state of equilibrium, the entropy of the system reaches a maximum and remains constant, i.e. dS = 0. Under isobaric-isothermal conditions, the driving force of the process, the Gibbs energy, at equilibrium takes a minimum value and does not change further, i.e. dG = 0.
The concentrations of participants in the reaction in a state of equilibrium are called equilibrium. As a rule, they are denoted by the formulas of the corresponding substances enclosed in square brackets, for example, the equilibrium concentration of ammonia is denoted in contrast to the initial, non-equilibrium concentration C^ NH ^.
Since the rates of direct and reverse processes in the state of equilibrium are equal, we equate the right parts of equations (4.44) and
- -^ i-
- (4.45), replacing the designation of concentrations: A: [A]""[B]" = ?[D] /; )