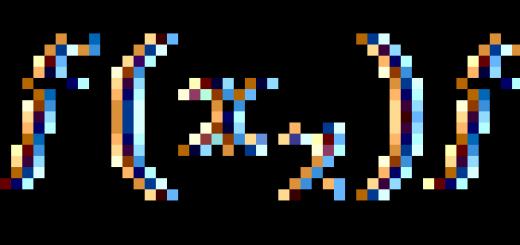4. Classification of proteins
Proteins and their main features
Proteins or proteins (which in Greek means “first” or “most important”) quantitatively predominate over all macromolecules present in a living cell, and make up more than half of the dry weight of most organisms. The concept of proteins as a class of compounds was formed in the 17th-19th centuries. During this period, substances with similar properties were isolated from various objects of the living world (seeds and juices of plants, muscles, blood, milk): they formed viscous solutions, coagulated when heated, the smell of burning wool was felt during combustion and ammonia was released. Since all these properties were previously known for egg white, the new class of compounds was called proteins. After the appearance at the beginning of the XIX century. More advanced methods of analysis of substances determined the elemental composition of proteins. They found C, H, O, N, S. By the end of the 19th century. More than 10 amino acids have been isolated from proteins. Based on the results of studying the products of protein hydrolysis, the German chemist E. Fischer (1852-1919) suggested that proteins are built from amino acids.
As a result of Fisher's work, it became clear that proteins are linear polymers of a-amino acids connected to each other by an amide (peptide) bond, and the whole variety of representatives of this class of compounds could be explained by differences in the amino acid composition and the order of alternation of different amino acids in the polymer chain.
The first studies of proteins were carried out with complex protein mixtures, for example: with blood serum, egg white, extracts of plant and animal tissues. Later, methods for isolating and purifying proteins were developed, such as precipitation, dialysis, chromatography on cellulose and other hydrophilic ion exchangers, gel filtration, and electrophoresis. Let's take a closer look at these methods in laboratory work and seminar session.
On the present stage The main areas of study of proteins are the following:
¨ study of the spatial structure of individual proteins;
¨ study of the biological functions of different proteins;
¨ study of the mechanisms of functioning of individual proteins (at the level of individual atoms, atomic groups of a protein molecule).
All these stages are interrelated, because one of the main tasks of biochemistry is precisely to understand how the amino acid sequences of different proteins enable them to perform various functions.
Biological functions of proteins
Enzymes - they are biological catalysts, the most diverse and numerous class of proteins. Almost all chemical reactions involving organic biomolecules present in the cell are catalyzed by enzymes. To date, more than 2000 different enzymes have been discovered.
Transport proteins- Transport proteins in blood plasma bind and carry specific molecules or ions from one organ to another. For example, hemoglobin, contained in erythrocytes, when passing through the lungs, it binds oxygen and delivers it to peripheral tissues, where oxygen is released. The blood plasma contains lipoproteins that transport lipids from the liver to other organs. In cell membranes, there is another type of cellular transport proteins that can bind certain molecules (eg, glucose) and transport them through the membrane into the cell.
Dietary and storage proteins. The best-known examples of such proteins are wheat, corn, and rice seed proteins. Dietary proteins are egg albumin- the main component of egg white, casein is the main protein in milk.
Contractile and motor proteins.Actin and myosin- proteins that function in the contractile system of skeletal muscle, as well as in many non-muscle tissues.
Structural proteins.Collagen- the main component of cartilage and tendons. This protein has a very high tensile strength. Bundles contain elastin- a structural protein capable of stretching in two dimensions. Hair, nails are composed almost exclusively of durable insoluble protein - keratin. The main component of silk threads and cobwebs is the protein fibroin.
protective proteins. Immunoglobulins or antibodies are specialized cells produced in lymphocytes. They have the ability to recognize viruses or foreign molecules that have entered the body of bacteria, and then launch a system to neutralize them. fibrinogen and thrombin- proteins involved in the process of blood clotting, they protect the body from blood loss when the vascular system is damaged.
regulatory proteins. Some proteins are involved in the regulation of cellular activity. These include many hormones such as insulin (regulates glucose metabolism).
Protein classification
By solubility
Albumins. Soluble in water and saline solutions.
Globulins. Slightly soluble in water, but highly soluble in saline solutions.
Prolamins. Soluble in 70-80% ethanol, insoluble in water and absolute alcohol. Rich in arginine.
Histones. Soluble in saline solutions.
Scleroproteins. Insoluble in water and saline solutions. The content of glycine, alanine, proline is increased.
The shape of the molecules
Based on the ratio of the axes (longitudinal and transverse), two large classes of proteins can be distinguished. At globular proteins the ratio is less than 10 and in most cases does not exceed 3-4. They are characterized by compact packing of polypeptide chains. Examples of globular proteins: many enzymes, insulin, globulin, plasma proteins, hemoglobin.
fibrillar proteins, in which the ratio of the axes exceeds 10, consist of bundles of polypeptide chains spirally wound on top of each other and interconnected by transverse covalent or hydrogen bonds (keratin, myosin, collagen, fibrin).
Physical properties of proteins
On the physical properties of proteins such as ionization,hydration, solubility various methods for isolating and purifying proteins are based.
Since proteins contain ionogenic, i.e. ionizable amino acid residues (arginine, lysine, glutamic acid, etc.), therefore, they are polyelectrolytes. With acidification, the degree of ionization of anionic groups decreases, while that of cationic groups increases; with alkalization, the opposite pattern is observed. At a certain pH, the number of negatively and positively charged particles becomes the same, this state is called isoelectric(the total charge of the molecule is zero). The pH value at which a protein is in an isoelectric state is called isoelectric point and denote pI. One of the methods for their separation is based on the different ionization of proteins at a certain pH value - the method electrophoresis.
Polar groups of proteins (ionic and nonionic) are able to interact with water and hydrate. The amount of water associated with protein reaches 30-50 g per 100 g of protein. There are more hydrophilic groups on the surface of the protein. Solubility depends on the number of hydrophilic groups in the protein, on the size and shape of the molecules, and on the magnitude of the total charge. The combination of all these physical properties of the protein makes it possible to use the method molecular sieves or gel filtration to separate proteins. Method dialysis It is used to purify proteins from low molecular weight impurities and is based on the large size of protein molecules.
The solubility of proteins also depends on the presence of other solutes, such as neutral salts. At high concentrations of neutral salts, proteins precipitate, and for precipitation ( salting out) different proteins require different concentrations of salt. This is due to the fact that charged protein molecules adsorb ions of opposite charge. As a result, the particles lose their charges and electrostatic repulsion, resulting in protein precipitation. The salting out method can be used to fractionate proteins.
Primary structure of proteins
Primary structure of a protein name the composition and sequence of amino acid residues in a protein molecule. Amino acids in a protein are linked by peptide bonds.
All molecules of a given individual protein are identical in amino acid composition, sequence of amino acid residues, and length of the polypeptide chain. Establishing the sequence of the amino acid sequence of proteins is a time-consuming task. We will discuss this topic in more detail at the seminar. Insulin was the first protein to have its amino acid sequence determined. Bovine insulin has a molar mass of about 5700. Its molecule consists of two polypeptide chains: an A chain containing 21 a.a., and a B chain containing 30 a.k., these two chains are connected by two disulfide (-S-S-) connections. Even small changes in the primary structure can significantly change the properties of a protein. The disease sickle cell anemia is the result of a change in just 1 amino acid in the b-chain of hemoglobin (Glu ® Val).
Species specificity of the primary structure
When studying amino acid sequences homologous proteins isolated from different species, several important conclusions were made. Homologous proteins are those proteins that perform the same functions in different species. An example is hemoglobin: in all vertebrates, it performs the same function associated with the transport of oxygen. Homologous proteins of different species usually have polypeptide chains of the same or nearly the same length. In the amino acid sequences of homologous proteins, the same amino acids are always found in many positions - they are called invariant residues. At the same time, significant differences are observed in other positions of proteins: in these positions, amino acids vary from species to species; such amino acid residues are called variable. The whole set of similar features in the amino acid sequences of homologous proteins is combined into the concept sequence homology. The presence of such homology suggests that the animals from which the homologous proteins were isolated share a common evolutionary origin. An interesting example is a complex protein - cytochrome c- mitochondrial protein involved as an electron carrier in the processes of biological oxidation. M » 12500, contains » 100 a.a. A.K. were installed. sequences for 60 species. 27 a.c. - are the same, which indicates that all these residues play an important role in determining the biological activity of cytochrome c. The second important conclusion drawn from the analysis of amino acid sequences is that the number of residues by which cytochromes differ from any two species is proportional to the phylogenetic difference between these species. For example, the molecules of cytochrome c from a horse and yeast differ by 48 a.a., in duck and chicken - by 2 a.a., in chicken and turkey they do not differ. Information on the number of differences in the amino acid sequences of homologous proteins from different species is used to build evolutionary maps that reflect the successive stages of the emergence and development of various animal and plant species in the evolutionary process.
Secondary structure of proteins
- this is the packing of a protein molecule in space without taking into account the influence of side substituents. There are two types of secondary structure: a-helix and b-structure (folded layer). Let us dwell in more detail on the consideration of each type of secondary structure.
a-Spiral is a right helix with the same pitch equal to 3.6 amino acid residues. The a-helix is stabilized by intramolecular hydrogen bonds that occur between the hydrogen atoms of one peptide bond and the oxygen atoms of the fourth peptide bond.
![]()
The side substituents are located perpendicular to the plane of the a-helix.
 |
That. the properties of a given protein are determined by the properties of the side groups of amino acid residues that are part of a particular protein. If the side substituents are hydrophobic, then the protein having the a-helix structure is also hydrophobic. An example of such a protein is the keratin protein that makes up hair.
As a result, it turns out that the a-helix is permeated with hydrogen bonds and is a very stable structure. In the formation of such a spiral, two tendencies work:
¨ the molecule tends to a minimum of energy, i.e. to the formation of the largest number of hydrogen bonds;
¨ due to the rigidity of the peptide bond, only the first and fourth peptide bonds can approach each other in space.
AT folded layer peptide chains are arranged parallel to each other, forming a figure similar to a sheet folded like an accordion. There can be a large number of peptide chains interacting with each other by hydrogen bonds. The chains are arranged antiparallel.
 |
The more peptide chains that make up the folded layer, the stronger the protein molecule.
Let us compare the properties of the protein materials of wool and silk and explain the difference in the properties of these materials in terms of the structure of the proteins of which they are composed.
Keratin - wool protein - has an a-helix secondary structure. Woolen thread is not as strong as silk, it stretches easily when wet. This property is explained by the fact that when a load is applied, the hydrogen bonds break and the helix stretches.
Fibroin - silk protein - has a secondary b-structure. The silk thread does not stretch and is very tear-resistant. This property is explained by the fact that in the folded layer many peptide chains interact with each other by hydrogen bonds, which makes this structure very strong.
Amino acids differ in their ability to participate in the formation of a-helices and b-structures. Glycine, aspargine, tyrosine are rarely found in a-helices. Proline destabilizes a-helical structure. Explain why? The composition of b-structures includes glycine, almost no proline, glutamic acid, aspargine, histidine, lysine, serine.
The structure of one protein may contain sections of b-structures, a-helices, and irregular sections. In irregular regions, the peptide chain can relatively easily bend and change conformation, while the helix and the folded layer are fairly rigid structures. The content of b-structures and a-helices in different proteins is not the same.
Tertiary structure of proteins
determined by the interaction of the side substituents of the peptide chain. For fibrillar proteins, it is difficult to identify general patterns in the formation of tertiary structures. As for globular proteins, such regularities exist, and we will consider them. The tertiary structure of globular proteins is formed by additional folding of the peptide chain containing b-structures, a-helices and irregular regions, so that the hydrophilic side groups of amino acid residues are on the surface of the globule, and the hydrophobic side groups are hidden deep into the globule, sometimes forming a hydrophobic pocket.
Forces that stabilize the tertiary structure of a protein.
Electrostatic interaction between differently charged groups, the extreme case is ionic interactions.
Hydrogen bonds arising between the side groups of the polypeptide chain.
Hydrophobic interactions.
covalent interactions(formation of a disulfide bond between two cysteine residues to form cystine). The formation of disulfide bonds leads to the fact that the remote regions of the polypeptide molecule approach each other and are fixed. Disulfide bonds are broken by reducing agents. This property is used to perm hair, which is almost entirely a keratin protein, riddled with disulfide bonds.
The nature of the spatial packing is determined by the amino acid composition and the alternation of amino acids in the polypeptide chain (primary structure). Therefore, each protein has only one spatial structure corresponding to its primary structure. Small changes in the conformation of protein molecules occur when interacting with other molecules. These changes sometimes play a huge role in the functioning of protein molecules. So, when an oxygen molecule is attached to hemoglobin, the conformation of the protein changes somewhat, which leads to the effect of cooperative interaction when the remaining three oxygen molecules are attached. Such a change in the conformation in underlies the theory of inducing correspondence in explaining the group specificity of some enzymes.
In addition to the covalent disulfide bond, all other bonds stabilizing the tertiary structure are inherently weak and easily destroyed. At break a large number bonds that stabilize the spatial structure of the protein molecule, the ordered conformation, unique for each protein, is broken, and the biological activity of the protein is often lost. This change in spatial structure is called denaturation.
Protein function inhibitors
Considering that different ligands differ in Kb, it is always possible to choose a substance similar in structure to the natural ligand, but having a higher Kb value with a given protein. For example, CO has a K St 100 times greater than O 2 with hemoglobin, so 0.1% CO in the air is enough to block a large number of hemoglobin molecules. Many medicines work on the same principle. For example, dithylin.

Acetylcholine is a mediator for the transmission of nerve impulses to the muscle. Ditilin blocks the receptor protein to which acetylcholine binds and creates the effect of paralysis.
9. Connection between the structure of proteins and their functions on the example of hemoglobin and myoglobin
Transport of carbon dioxide
Hemoglobin not only carries oxygen from the lungs to peripheral tissues, but also accelerates the transport of CO 2 from tissues to the lungs. Hemoglobin binds CO 2 immediately after the release of oxygen (» 15% of total CO 2). In erythrocytes, an enzymatic process of formation of carbonic acid from CO 2 coming from tissues occurs: CO 2 + H 2 O \u003d H 2 CO 3. Carbonic acid quickly dissociates into HCO 3 - and H +. To prevent a dangerous increase in acidity, there must be a buffer system capable of absorbing excess protons. Hemoglobin binds two protons for every four oxygen molecules released and determines the buffering capacity of the blood. In the lungs, the process is reversed. The released protons bind to the bicarbonate ion to form carbonic acid, which, under the action of the enzyme, is converted into CO 2 and water, CO 2 is exhaled. Thus, the binding of O 2 is closely associated with the exhalation of CO 2 . This reversible phenomenon is known as Bohr effect. Myoglobin does not exhibit the Bohr effect.
Isofunctional proteins
A protein that performs a specific function in a cell can be represented by several forms - isofunctional proteins, or isoenzymes. Although such proteins perform the same function, they differ in the binding constant, which leads to some differences in functional terms. For example, several forms of hemoglobin were found in human erythrocytes: HbA (96%), HbF (2%), HbA 2 (2%). All hemoglobins are tetramers built from protomers a, b, g, d (HbA - a 2 b 2, HbF - a 2 g 2, HbA 2 - a 2 d 2). All protomers are similar to each other in the primary structure, and a very large similarity is observed in the secondary and tertiary structures. All forms of hemoglobin are designed to carry oxygen to tissue cells, but HbF, for example, has a greater affinity for oxygen than HbA. HbF is characteristic of the embryonic stage of human development. It is able to take oxygen from HbA, which ensures a normal supply of oxygen to the fetus.
Isoproteins are the result of having more than one structural gene in a species' gene pool.
PROTEINS: STRUCTURE, PROPERTIES AND FUNCTIONS
1. Proteins and their main features
2. Biological functions of proteins
3. Amino acid composition of proteins
4. Classification of proteins
5. Physical properties of proteins
6. Structural organization of protein molecules (primary, secondary, tertiary structures)
Proteins- these are high-molecular (molecular weight varies from 5-10 thousand to 1 million or more) natural polymers, the molecules of which are built from amino acid residues connected by an amide (peptide) bond.
Proteins are also called proteins (Greek "protos" - the first, important). The number of amino acid residues in a protein molecule varies greatly and sometimes reaches several thousand. Each protein has its own sequence of amino acid residues.
Proteins perform a variety of biological functions: catalytic (enzymes), regulatory (hormones), structural (collagen, fibroin), motor (myosin), transport (hemoglobin, myoglobin), protective (immunoglobulins, interferon), spare (casein, albumin, gliadin) other.
Proteins are the basis of biomembranes, the most important part of the cell and cellular components. They play a key role in the life of the cell, forming, as it were, the material basis of its chemical activity.
An exceptional property of protein - self-organization structure, i.e., its ability to spontaneously create a specific spatial structure peculiar only to a given protein. Essentially, all the activities of the organism (development, movement, performance by it various functions and much more) is associated with protein substances. It is impossible to imagine life without proteins.
Proteins are the most important component of human and animal food, a supplier of essential amino acids.
The structure of proteins
In the spatial structure of proteins great importance has the character of radicals (residues) R- in amino acid molecules. Nonpolar amino acid radicals are usually located inside the protein macromolecule and cause hydrophobic interactions; polar radicals containing ionogenic (ion-forming) groups are usually located on the surface of a protein macromolecule and characterize electrostatic (ionic) interactions. Polar nonionic radicals (for example, containing alcohol OH groups, amide groups) can be located both on the surface and inside the protein molecule. They participate in the formation of hydrogen bonds.
In protein molecules, α-amino acids are interconnected by peptide (-CO-NH-) bonds:
The polypeptide chains constructed in this way or individual sections within the polypeptide chain can in some cases be additionally interconnected by disulfide (-S-S-) bonds or, as they are often called, disulfide bridges.
An important role in creating the structure of proteins is played by ionic (salt) and hydrogen bonds, as well as hydrophobic interaction - a special type of contact between the hydrophobic components of protein molecules in an aqueous medium. All these bonds have different strengths and provide the formation of a complex, large protein molecule.
Despite the difference in the structure and functions of protein substances, their elemental composition fluctuates slightly (in % of dry mass): carbon - 51-53; oxygen - 21.5-23.5; nitrogen - 16.8-18.4; hydrogen - 6.5-7.3; sulfur - 0.3-2.5.
Some proteins contain small amounts of phosphorus, selenium and other elements.
The sequence of amino acid residues in a polypeptide chain is called primary structure of the protein.
A protein molecule may consist of one or more polypeptide chains, each containing a different number of amino acid residues. Given the number of their possible combinations, it can be said that the variety of proteins is almost limitless, but not all of them exist in nature.
The total number of different types of proteins in all types of living organisms is 10 11 -10 12 . For proteins, the structure of which is extremely complex, in addition to the primary one, there are also higher levels of structural organization: secondary, tertiary, and sometimes quaternary structures.
secondary structure possesses most of the proteins, however, not always throughout the polypeptide chain. Polypeptide chains with a certain secondary structure can be arranged differently in space.
In formation tertiary structure, in addition to hydrogen bonds, ionic and hydrophobic interactions play an important role. According to the nature of the "packaging" of the protein molecule, globular, or spherical, and fibrillar, or filamentous, proteins (Table 12).
For globular proteins, it is more characteristic a-helical structure, the spirals are bent, "folded". The macromolecule has a spherical shape. They dissolve in water and saline solutions to form colloidal systems. Most animal, plant, and microorganism proteins are globular proteins.


For fibrillar proteins, a filamentous structure is more characteristic. They generally do not dissolve in water. Fibrillar proteins usually perform structure-forming functions. Their properties (strength, ability to stretch) depend on the way the polypeptide chains are packed. An example of fibrillar proteins are myosin, keratin. In some cases, individual protein subunits form complex ensembles with the help of hydrogen bonds, electrostatic and other interactions. In this case, it forms quaternary structure proteins.
Blood hemoglobin is an example of a protein with a quaternary structure. Only with such a structure does it perform its functions - binding oxygen and transporting it to tissues and organs.
However, it should be noted that the primary structure plays an exceptional role in the organization of higher protein structures.
Protein classification
There are several classifications of proteins:
- According to the degree of difficulty (simple and complex).
- By the shape of the molecules (globular and fibrillar proteins).
- By solubility in individual solvents (water-soluble, soluble in dilute saline solutions - albumins, alcohol-soluble - prolamins, soluble in dilute alkalis and acids - glutelins).
- According to the functions performed (for example, storage proteins, skeletal, etc.).
Protein properties
Proteins are amphoteric electrolytes. At a certain pH value of the medium (it is called the isoelectric point), the number of positive and negative charges in the protein molecule is the same. This is one of the main properties of protein. Proteins at this point are electrically neutral, and their solubility in water is the lowest. The ability of proteins to reduce solubility when their molecules become electrically neutral is used for isolation from solutions, for example, in the technology of obtaining protein products.
Hydration. The process of hydration means the binding of water by proteins, while they exhibit hydrophilic properties: they swell, their mass and volume increase. The swelling of individual proteins depends solely on their structure. The hydrophilic amide (-CO-NH-, peptide bond), amine (-NH 2) and carboxyl (-COOH) groups present in the composition and located on the surface of the protein macromolecule attract water molecules, strictly orienting them on the surface of the molecule. The hydration (water) shell surrounding the protein globules prevents aggregation and sedimentation and, consequently, contributes to the stability of protein solutions. At the isoelectric point, proteins have the least ability to bind water; the hydration shell around the protein molecules is destroyed, so they combine to form large aggregates. Aggregation of protein molecules also occurs during their dehydration with the help of some organic solvents, for example, ethyl alcohol. This leads to the precipitation of proteins. When the pH of the medium changes, the protein macromolecule becomes charged, and its hydration capacity changes.
With limited swelling, concentrated protein solutions form complex systems called jelly.
The jellies are not fluid, elastic, have plasticity, a certain mechanical strength, and are able to maintain their shape. Globular proteins can be completely hydrated, dissolve in water (for example, milk proteins), forming solutions with a low concentration. The hydrophilic properties of proteins, i.e. their ability to swell, form jellies, stabilize suspensions, emulsions and foams, are of great importance in biology and the food industry. A very mobile jelly, built mainly from protein molecules, is the cytoplasm - raw gluten isolated from wheat dough; it contains up to 65% water. The different hydrophilicity of gluten proteins is one of the signs that characterize the quality of wheat grain and the flour obtained from it (the so-called strong and weak wheat). The hydrophilicity of grain and flour proteins plays an important role in the storage and processing of grain, in baking. The dough, which is obtained in the baking industry, is a protein swollen in water, a concentrated jelly containing starch grains.
Protein denaturation. During denaturation, under the influence of external factors (temperature, mechanical action, the action of chemical agents, and a number of other factors), a change occurs in the secondary, tertiary, and quaternary structures of the protein macromolecule, i.e., its native spatial structure. The primary structure and, consequently, the chemical composition of the protein do not change. Physical properties change: solubility decreases, ability to hydrate, biological activity is lost. The shape of the protein macromolecule changes, aggregation occurs. At the same time, the activity of some chemical groups increases, the effect of proteolytic enzymes on proteins is facilitated, and, consequently, it is more easily hydrolyzed.
In food technology, thermal denaturation of proteins is of particular practical importance, the degree of which depends on temperature, duration of heating and humidity. This must be remembered when developing modes of heat treatment of food raw materials, semi-finished products, and sometimes finished products. The processes of thermal denaturation play a special role in blanching vegetable raw materials, drying grain, baking bread, and obtaining pasta. Protein denaturation can also be caused by mechanical action (pressure, rubbing, shaking, ultrasound). Finally, the action of chemical reagents (acids, alkalis, alcohol, acetone) leads to the denaturation of proteins. All these techniques are widely used in food and biotechnology.
Foaming. The process of foaming is understood as the ability of proteins to form highly concentrated liquid-gas systems, called foams. The stability of the foam, in which the protein is a blowing agent, depends not only on its nature and concentration, but also on temperature. Proteins as foaming agents are widely used in the confectionery industry (marshmallow, marshmallow, soufflé). The structure of the foam has bread, and this affects its taste.
Protein molecules under the influence of a number of factors can be destroyed or interact with other substances to form new products. For the food industry, two important processes can be distinguished:
1) hydrolysis of proteins under the action of enzymes;
2) interaction of amino groups of proteins or amino acids with carbonyl groups of reducing sugars.
Under the influence of protease enzymes that catalyze the hydrolytic cleavage of proteins, the latter break down into simpler products (poly- and dipeptides) and ultimately into amino acids. The rate of protein hydrolysis depends on its composition, molecular structure, enzyme activity, and conditions.
Protein hydrolysis. The hydrolysis reaction with the formation of amino acids in general terms can be written as follows:

Combustion. Proteins burn to form nitrogen carbon dioxide and water, as well as some other substances. Burning is accompanied by the characteristic smell of burnt feathers.
Color reactions for proteins. For qualitative definition proteins use the following reactions:
1) xantoprotein, at which the interaction of aromatic and heteroatomic cycles in the protein molecule with concentrated nitric acid occurs, accompanied by the appearance of a yellow color.

2) biuret, at which weakly alkaline solutions of proteins interact with a solution of copper sulfate (II) with the formation of complex compounds between Cu 2+ ions and polypeptides. The reaction is accompanied by the appearance of a violet-blue color.

Squirrels— macromolecular organic compounds, consisting of amino acid residues connected in a long chain by a peptide bond.
The composition of the proteins of living organisms includes only 20 types of amino acids, all of which are alpha-amino acids, and the amino acid composition of proteins and their order of connection with each other are determined by the individual. genetic code living organism.
One of the features of proteins is their ability to spontaneously form spatial structures characteristic only for this particular protein.
Due to the specificity of their structure, proteins can have a variety of properties. For example, proteins having a globular quaternary structure, in particular chicken egg protein, dissolve in water to form colloidal solutions. Proteins with a fibrillar quaternary structure do not dissolve in water. Fibrillar proteins, in particular, form nails, hair, cartilage.
Chemical properties of proteins
Hydrolysis
All proteins are capable of undergoing hydrolysis. In the case of complete hydrolysis of proteins, a mixture of α-amino acids is formed:
Protein + nH 2 O => mixture of α-amino acids
Denaturation
The destruction of the secondary, tertiary and quaternary structures of a protein without destroying its primary structure is called denaturation. Protein denaturation can proceed under the action of solutions of sodium, potassium or ammonium salts - such denaturation is reversible:
Denaturation occurring under the influence of radiation (for example, heating) or processing of protein with salts of heavy metals is irreversible:
So, for example, irreversible protein denaturation is observed during the heat treatment of eggs during their preparation. As a result of egg white denaturation, its ability to dissolve in water with the formation of a colloidal solution disappears.
Qualitative reactions to proteins
Biuret reaction
If 10% sodium hydroxide solution is added to a solution containing protein, and then a small amount of 1% copper sulfate solution, a violet color will appear.
protein solution + NaOH (10% solution) + СuSO 4 = violet color
xantoprotein reaction
protein solutions when boiled with concentrated nitric acid turn yellow:
protein solution + HNO 3 (conc.) => yellow color
Biological functions of proteins
| catalytic | speed up various chemical reactions in living organisms | enzymes |
| structural | cell building material | collagen, cell membrane proteins |
| protective | protect the body from infections | immunoglobulins, interferon |
| regulatory | regulate metabolic processes | hormones |
| transport | transfer of vital substances from one part of the body to another | hemoglobin carries oxygen |
| energy | supply the body with energy | 1 gram of protein can provide the body with 17.6 J of energy |
| motor (motor) | any motor function of the body | myosin (muscle protein) |
Squirrels - These are biopolymers consisting of α-amino acid residues interconnected by peptide bonds (-CO-NH-). Proteins are part of the cells and tissues of all living organisms. Protein molecules contain 20 different amino acid residues.
protein structure
Proteins have an inexhaustible variety of structures.
Primary structure of a protein is the sequence of amino acid units in a linear polypeptide chain.
secondary structure- this is a spatial configuration of a protein molecule, resembling a helix, which is formed as a result of twisting the polypeptide chain due to hydrogen bonds between groups: CO and NH.
Tertiary structure- this is the spatial configuration that the polypeptide chain twisted into a spiral takes.
Quaternary structure are polymeric formations of several protein macromolecules.
Physical Properties
The properties of proteins are very diverse, which they perform. Some proteins dissolve in water, forming, as a rule, colloidal solutions (for example, egg white); others dissolve in dilute salt solutions; others are insoluble (for example, proteins of integumentary tissues).
Chemical properties
Denaturation- destruction of the secondary, tertiary structure of the protein under the influence of various factors: temperature, the action of acids, salts heavy metals, alcohols, etc.
During denaturation under the influence of external factors (temperature, mechanical action, the action of chemical agents and other factors), a change occurs in the secondary, tertiary and quaternary structures of the protein macromolecule, that is, its native spatial structure. The primary structure and, consequently, the chemical composition of the protein do not change. are changing physical properties: reduced solubility, ability to hydrate, loss of biological activity. The shape of the protein macromolecule changes, aggregation occurs. At the same time, the activity of some groups increases, the effect of proteolytic enzymes on proteins is facilitated, and, consequently, it is more easily hydrolyzed.
In food technology, thermal denaturation of proteins is of particular practical importance, the degree of which depends on temperature, heating time, and humidity. This must be remembered when developing modes of heat treatment of food raw materials, semi-finished products, and sometimes finished products. Thermal denaturation processes play a special role in blanching plant materials, drying grain, baking bread, and obtaining pasta. Protein denaturation can also be caused by mechanical action (pressure, rubbing, shaking, ultrasound). The action of chemical reagents (acids, alkalis, alcohol, acetone) leads to the denaturation of proteins. All these techniques are widely used in food and biotechnology.
Qualitative reactions to proteins:
a) When burning protein - the smell of burnt feathers.
b) Protein + HNO 3 → yellow color
c) Protein solution + NaOH + CuSO 4 → violet color
Hydrolysis
Protein + H 2 O → a mixture of amino acids
Functions of proteins in nature:
catalytic (enzymes);
Regulatory (hormones);
Structural (wool keratin, silk fibroin, collagen);
motor (actin, myosin);
transport (hemoglobin);
Spare (casein, egg albumin);
protective (immunoglobulins), etc.
Hydration
The process of hydration means the binding of water by proteins, while they exhibit hydrophilic properties: they swell, their mass and volume increase. Protein swelling is accompanied by its partial dissolution. The hydrophilicity of individual proteins depends on their structure. The hydrophilic amide (–CO–NH–, peptide bond), amine (NH 2), and carboxyl (COOH) groups present in the composition and located on the surface of the protein macromolecule attract water molecules, strictly orienting them to the surface of the molecule. Surrounding the protein globules, the hydrate (water) shell prevents the stability of protein solutions. At the isoelectric point, proteins have the least ability to bind water; the hydration shell around the protein molecules is destroyed, so they combine to form large aggregates. Aggregation of protein molecules also occurs when they are dehydrated with some organic solvents, such as ethyl alcohol. This leads to the precipitation of proteins. When the pH of the medium changes, the protein macromolecule becomes charged, and its hydration capacity changes.
With limited swelling, concentrated protein solutions form complex systems called jelly. The jellies are not fluid, elastic, have plasticity, a certain mechanical strength, and are able to maintain their shape. Globular proteins can be completely hydrated by dissolving in water (for example, milk proteins), forming solutions with a low concentration. The hydrophilic properties of proteins are of great importance in biology and the food industry. A very mobile jelly, built mainly of protein molecules, is the cytoplasm - the semi-liquid contents of the cell. Highly hydrated jelly is raw gluten isolated from wheat dough and contains up to 65% water. Hydrophilicity, the main quality of wheat grain, grain proteins and flour, plays an important role in the storage and processing of grain, in baking. The dough, which is obtained in the bakery industry, is a protein swollen in water, a concentrated jelly containing starch grains.
Foaming
The foaming process is the ability of proteins to form highly concentrated liquid-gas systems called foams. The stability of the foam, in which the protein is a foaming agent, depends not only on its nature and concentration, but also on temperature. Proteins are widely used as foaming agents in the confectionery industry (marshmallow, marshmallow, soufflé). Bread has a foam structure, and this affects its taste properties.
Combustion
Proteins burn with the formation of nitrogen, carbon dioxide and water, as well as some other substances. Burning is accompanied by the characteristic smell of burnt feathers.
color reactions.
- Xantoprotein - interaction of aromatic and heteroatomic cycles in a protein molecule with concentrated nitric acid occurs, accompanied by the appearance of a yellow color;
- Biuret - there is an interaction of weakly alkaline solutions of proteins with a solution of copper (II) sulfate with the formation of complex compounds between Cu 2+ ions and polypeptides. The reaction is accompanied by the appearance of a violet-blue color;
- when proteins are heated with alkali in the presence of lead salts, a black precipitate forms, which contains sulfur.
The content of the article
PROTEINS (Article 1)- a class of biological polymers present in every living organism. With the participation of proteins, the main processes that ensure the vital activity of the body take place: respiration, digestion, muscle contraction, transmission of nerve impulses. Bone tissue, skin, hair, horn formations of living beings are composed of proteins. For most mammals, the growth and development of the organism occurs due to products containing proteins as a food component. The role of proteins in the body and, accordingly, their structure is very diverse.
The composition of proteins.
All proteins are polymers, the chains of which are assembled from fragments of amino acids. Amino acids are organic compounds containing in their composition (in accordance with the name) an NH 2 amino group and an organic acid, i.e. carboxyl, COOH group. Of the entire variety of existing amino acids (theoretically, the number of possible amino acids is unlimited), only those that have only one carbon atom between the amino group and the carboxyl group participate in the formation of proteins. In general, the amino acids involved in the formation of proteins can be represented by the formula: H 2 N–CH(R)–COOH. The R group attached to the carbon atom (the one between the amino and carboxyl groups) determines the difference between the amino acids that make up proteins. This group can consist only of carbon and hydrogen atoms, but more often contains, in addition to C and H, various functional (capable of further transformations) groups, for example, HO-, H 2 N-, etc. There is also an option when R = H.
The organisms of living beings contain more than 100 different amino acids, however, not all are used in the construction of proteins, but only 20, the so-called "fundamental". In table. 1 shows their names (most of the names have developed historically), the structural formula, as well as the widely used abbreviation. All structural formulas are arranged in the table so that the main fragment of the amino acid is on the right.
| Name | Structure | Designation |
| GLYCINE | GLI | |
| ALANIN | ALA | |
| VALIN | SHAFT | |
| LEUCINE | LEI | |
| ISOLEUCINE | ILE | |
| SERIN | SER | |
| THREONINE | TRE | |
| CYSTEINE | CIS | |
| METIONINE | MET | |
| LYSINE | LIZ | |
| ARGININE | AWG | |
| ASPARAGIC ACID | ASN | |
| ASPARAGIN | ASN | |
| GLUTAMIC ACID | GLU | |
| GLUTAMINE | GLN | |
| phenylalanine | hair dryer | |
| TYROSINE | TIR | |
| tryptophan | THREE | |
| HISTIDINE | GIS | |
| PROLINE | PRO | |
| In international practice, the abbreviated designation of the listed amino acids using Latin three-letter or one-letter abbreviations is accepted, for example, glycine - Gly or G, alanine - Ala or A. | ||
Among these twenty amino acids (Table 1), only proline contains an NH group (instead of NH 2) next to the COOH carboxyl group, since it is part of the cyclic fragment.
Eight amino acids (valine, leucine, isoleucine, threonine, methionine, lysine, phenylalanine and tryptophan), placed in the table on a gray background, are called essential, since the body must constantly receive them with protein food for normal growth and development.
A protein molecule is formed as a result of the sequential connection of amino acids, while the carboxyl group of one acid interacts with the amino group of the neighboring molecule, as a result, a –CO–NH– peptide bond is formed and a water molecule is released. On fig. 1 shows the serial connection of alanine, valine and glycine.
Rice. one SERIAL CONNECTION OF AMINO ACIDS during the formation of a protein molecule. The path from the terminal amino group H 2 N to the terminal carboxyl group COOH was chosen as the main direction of the polymer chain.
To compactly describe the structure of a protein molecule, the abbreviations for amino acids (Table 1, third column) involved in the formation of the polymer chain are used. The fragment of the molecule shown in Fig. 1 is written as follows: H 2 N-ALA-VAL-GLY-COOH.
Protein molecules contain from 50 to 1500 amino acid residues (shorter chains are called polypeptides). The individuality of a protein is determined by the set of amino acids that make up the polymer chain and, no less important, by the order of their alternation along the chain. For example, the insulin molecule consists of 51 amino acid residues (it is one of the shortest chain proteins) and consists of two interconnected parallel chains of unequal length. The sequence of amino acid fragments is shown in fig. 2.
Rice. 2 INSULIN MOLECULE, built from 51 amino acid residues, fragments of the same amino acids are marked with the corresponding background color. The cysteine amino acid residues (abbreviated designation CIS) contained in the chain form disulfide bridges -S-S-, which link two polymer molecules, or form jumpers within one chain.
Molecules of the amino acid cysteine (Table 1) contain reactive sulfhydride groups -SH, which interact with each other, forming disulfide bridges -S-S-. The role of cysteine in the world of proteins is special, with its participation, cross-links are formed between polymeric protein molecules.
The combination of amino acids into a polymer chain occurs in a living organism under the control of nucleic acids, it is they that provide a strict assembly order and regulate the fixed length of the polymer molecule ( cm. NUCLEIC ACIDS).
The structure of proteins.
The composition of the protein molecule, presented in the form of alternating amino acid residues (Fig. 2), is called the primary structure of the protein. Hydrogen bonds arise between the imino groups HN present in the polymer chain and the carbonyl groups CO ( cm. HYDROGEN BOND), as a result, the protein molecule acquires a certain spatial shape, called the secondary structure. The most common are two types of secondary structure in proteins.
The first option, called the α-helix, is implemented using hydrogen bonds within one polymer molecule. Geometric parameters of a molecule determined by bond lengths and bond angles, are such that the formation of hydrogen bonds is possible for groups H-N and C=O, between which there are two peptide fragments H-N-C=O (Fig. 3).
The composition of the polypeptide chain shown in fig. 3 is written in abbreviated form as follows:
H 2 N-ALA VAL-ALA-LEY-ALA-ALA-ALA-ALA-VAL-ALA-ALA-ALA-COOH.
As a result of the contracting action of hydrogen bonds, the molecule takes the form of a helix - the so-called α-helix, it is depicted as a curved helical ribbon passing through the atoms that form the polymer chain (Fig. 4)
Rice. 4 3D MODEL OF A PROTEIN MOLECULE in the form of an α-helix. Hydrogen bonds are shown as green dotted lines. The cylindrical shape of the spiral is visible at a certain angle of rotation (hydrogen atoms are not shown in the figure). The color of individual atoms is given in accordance with international rules, which recommend black for carbon atoms, blue for nitrogen, red for oxygen, and yellow for sulfur (white color is recommended for hydrogen atoms not shown in the figure, in this case the entire structure depicted on a dark background).
Another variant of the secondary structure, called the β-structure, is also formed with the participation of hydrogen bonds, the difference is that the H-N and C=O groups of two or more polymer chains located in parallel interact. Since the polypeptide chain has a direction (Fig. 1), variants are possible when the direction of the chains is the same (parallel β-structure, Fig. 5), or they are opposite (antiparallel β-structure, Fig. 6).
Polymer chains of various compositions can participate in the formation of the β-structure, while organic groups, framing the polymer chain (Ph, CH 2 OH, etc.), in most cases play a secondary role, the mutual arrangement of the H-N and C=O groups is of decisive importance. Since the H-N and C=O groups are directed in different directions relative to the polymer chain (up and down in the figure), it becomes possible for three or more chains to interact simultaneously.
The composition of the first polypeptide chain in Fig. 5:
H 2 N-LEI-ALA-FEN-GLI-ALA-ALA-COOH
The composition of the second and third chain:
H 2 N-GLY-ALA-SER-GLY-TRE-ALA-COOH
The composition of the polypeptide chains shown in fig. 6, the same as in Fig. 5, the difference is that the second chain has the opposite (in comparison with Fig. 5) direction.
It is possible to form a β-structure inside one molecule, when the chain fragment in a certain section turns out to be rotated by 180°, in this case, two branches of one molecule have the opposite direction, as a result, an antiparallel β-structure is formed (Fig. 7).
The structure shown in fig. 7 in a flat image, shown in fig. 8 in the form of a three-dimensional model. Sections of the β-structure are usually denoted in a simplified way by a flat wavy ribbon that passes through the atoms that form the polymer chain.
In the structure of many proteins, sections of the α-helix and ribbon-like β-structures alternate, as well as single polypeptide chains. Their mutual arrangement and alternation in the polymer chain is called the tertiary structure of the protein.
Methods for depicting the structure of proteins are shown below using the plant protein crambin as an example. Structural formulas of proteins, often containing up to hundreds of amino acid fragments, are complex, cumbersome and difficult to understand, so sometimes simplified structural formulas are used - without symbols chemical elements(Fig. 9, variant A), but at the same time retain the color of the valence strokes in accordance with international rules (Fig. 4). In this case, the formula is presented not in a flat, but in a spatial image, which corresponds to the real structure of the molecule. This method makes it possible, for example, to distinguish between disulfide bridges (similar to those in insulin, Fig. 2), phenyl groups in the side frame of the chain, etc. The image of molecules in the form of three-dimensional models (balls connected by rods) is somewhat clearer (Fig. 9, option B). However, both methods do not allow showing the tertiary structure, so the American biophysicist Jane Richardson proposed to represent α-structures as spirally twisted ribbons (see Fig. 4), β-structures as flat wavy ribbons (Fig. 8), and connecting them single chains - in the form of thin bundles, each type of structure has its own color. This method of depicting the tertiary structure of a protein is now widely used (Fig. 9, variant B). Sometimes, for greater information content, a tertiary structure and a simplified structural formula are shown together (Fig. 9, variant D). There are also modifications of the method proposed by Richardson: α-helices are depicted as cylinders, and β-structures are in the form of flat arrows indicating the direction of the chain (Fig. 9, option E). Less common is the method in which the entire molecule is depicted as a bundle, where unequal structures are distinguished by different colors, and disulfide bridges are shown as yellow bridges (Fig. 9, variant E).
Option B is the most convenient for perception, when, when depicting the tertiary structure, the structural features of the protein (amino acid fragments, their alternation order, hydrogen bonds) are not indicated, while it is assumed that all proteins contain “details” taken from a standard set of twenty amino acids ( Table 1). The main task in depicting a tertiary structure is to show the spatial arrangement and alternation of secondary structures.
Rice. nine VARIOUS VERSIONS OF IMAGE OF THE STRUCTURE OF THE CRUMBIN PROTEIN.
A is a structural formula in a spatial image.
B - structure in the form of a three-dimensional model.
B is the tertiary structure of the molecule.
G - a combination of options A and B.
E - simplified image of the tertiary structure.
E - tertiary structure with disulfide bridges.
The most convenient for perception is a three-dimensional tertiary structure (option B), freed from the details of the structural formula.
A protein molecule that has a tertiary structure, as a rule, takes on a certain configuration, which is formed by polar (electrostatic) interactions and hydrogen bonds. As a result, the molecule takes the form of a compact coil - globular proteins (globules, lat. ball), or filamentous - fibrillar proteins (fibra, lat. fiber).
An example of a globular structure is the protein albumin, the protein of a chicken egg belongs to the class of albumins. The polymeric chain of albumin is assembled mainly from alanine, aspartic acid, glycine, and cysteine, alternating in a certain order. The tertiary structure contains α-helices connected by single chains (Fig. 10).
Rice. ten GLOBULAR STRUCTURE OF ALBUMIN
An example of a fibrillar structure is the fibroin protein. They contain a large amount of glycine, alanine and serine residues (every second amino acid residue is glycine); cysteine residues containing sulfhydride groups are absent. Fibroin, the main component of natural silk and cobwebs, contains β-structures connected by single chains (Fig. 11).
Rice. eleven FIBRILLARY PROTEIN FIBROIN
The possibility of forming a tertiary structure of a certain type is inherent in the primary structure of the protein, i.e. determined in advance by the order of alternation of amino acid residues. From certain sets of such residues, α-helices predominantly arise (there are quite a lot of such sets), another set leads to the appearance of β-structures, single chains are characterized by their composition.
Some protein molecules, while retaining a tertiary structure, are able to combine into large supramolecular aggregates, while they are held together by polar interactions, as well as hydrogen bonds. Such formations are called the quaternary structure of the protein. For example, the protein ferritin, which consists mainly of leucine, glutamic acid, aspartic acid and histidine (ferricin contains all 20 amino acid residues in varying amounts) forms a tertiary structure of four parallel-laid α-helices. When molecules are combined into a single ensemble (Fig. 12), a quaternary structure is formed, which can include up to 24 ferritin molecules.
Fig.12 FORMATION OF THE QUATERNARY STRUCTURE OF THE GLOBULAR PROTEIN FERRITIN
Another example of supramolecular formations is the structure of collagen. It is a fibrillar protein whose chains are built mainly of glycine alternating with proline and lysine. The structure contains single chains, triple α-helices, alternating with ribbon-like β-structures stacked in parallel bundles (Fig. 13).
Fig.13 SUPRAMOLECULAR STRUCTURE OF COLLAGEN FIBRILLARY PROTEIN
Chemical properties of proteins.
Under the action of organic solvents, waste products of some bacteria (lactic acid fermentation) or with an increase in temperature, secondary and tertiary structures are destroyed without damaging its primary structure, as a result, the protein loses solubility and loses biological activity, this process is called denaturation, that is, the loss of natural properties, for example, the curdling of sour milk, the coagulated protein of a boiled chicken egg. At elevated temperatures, the proteins of living organisms (in particular, microorganisms) quickly denature. Such proteins are not able to participate in biological processes, as a result, microorganisms die, so boiled (or pasteurized) milk can last longer.
Peptide bonds H-N-C=O, forming the polymer chain of the protein molecule, are hydrolyzed in the presence of acids or alkalis, and the polymer chain breaks, which, ultimately, can lead to the original amino acids. Peptide bonds that are part of α-helices or β-structures are more resistant to hydrolysis and various chemical influences (compared to the same bonds in single chains). A more delicate disassembly of the protein molecule into its constituent amino acids is carried out in an anhydrous medium using hydrazine H 2 N–NH 2, while all amino acid fragments, except for the last one, form the so-called hydrazides carboxylic acids containing the C(O)–HN–NH2 fragment (Fig. 14).
Rice. fourteen. POLYPEPTIDE CLEAVAGE
Such an analysis can provide information about the amino acid composition of a protein, but it is more important to know their sequence in a protein molecule. One of the methods widely used for this purpose is the action of phenylisothiocyanate (FITC) on the polypeptide chain, which in an alkaline medium attaches to the polypeptide (from the end that contains the amino group), and when the reaction of the medium changes to acidic, it detaches from the chain, taking with it fragment of one amino acid (Fig. 15).
Rice. fifteen SEQUENTIAL POLYPEPTIDE Cleavage
Many special methods have been developed for such an analysis, including those that begin to “disassemble” a protein molecule into its constituent components, starting from the carboxyl end.
Cross disulfide bridges S-S (formed by the interaction of cysteine residues, Fig. 2 and 9) are cleaved, turning them into HS-groups by the action of various reducing agents. The action of oxidizing agents (oxygen or hydrogen peroxide) again leads to the formation of disulfide bridges (Fig. 16).
Rice. sixteen. Cleavage of disulfide bridges
To create additional cross-links in proteins, the reactivity of amino and carboxyl groups is used. More accessible for various interactions are the amino groups that are in the side frame of the chain - fragments of lysine, asparagine, lysine, proline (Table 1). When such amino groups interact with formaldehyde, the process of condensation occurs and cross-bridges –NH–CH2–NH– appear (Fig. 17).
Rice. 17 CREATION OF ADDITIONAL TRANSVERSAL BRIDGES BETWEEN PROTEIN MOLECULES.
The terminal carboxyl groups of the protein are able to react with complex compounds of some polyvalent metals (chromium compounds are more often used), and cross-links also occur. Both processes are used in leather tanning.
The role of proteins in the body.
The role of proteins in the body is diverse.
Enzymes(fermentatio lat. - fermentation), their other name is enzymes (en zumh greek. - in yeast) - these are proteins with catalytic activity, they are able to increase the speed of biochemical processes by thousands of times. Under the action of enzymes, the constituent components of food: proteins, fats and carbohydrates are broken down into simpler compounds, from which new macromolecules are then synthesized, which are necessary for a certain type of body. Enzymes also take part in many biochemical processes of synthesis, for example, in the synthesis of proteins (some proteins help to synthesize others). Cm. ENZYMES
Enzymes are not only highly efficient catalysts, but also selective (direct the reaction strictly in the given direction). In their presence, the reaction proceeds with almost 100% yield without the formation of by-products and, at the same time, the flow conditions are mild: normal Atmosphere pressure and body temperature. For comparison, the synthesis of ammonia from hydrogen and nitrogen in the presence of an activated iron catalyst is carried out at 400–500°C and a pressure of 30 MPa, the yield of ammonia is 15–25% per cycle. Enzymes are considered unsurpassed catalysts.
Intensive study of enzymes began in the middle of the 19th century; more than 2,000 different enzymes have now been studied; this is the most diverse class of proteins.
The names of enzymes are as follows: the name of the reagent with which the enzyme interacts, or the name of the catalyzed reaction, is added with the ending -aza, for example, arginase decomposes arginine (Table 1), decarboxylase catalyzes decarboxylation, i.e. elimination of CO 2 from the carboxyl group:
– COOH → – CH + CO 2
Often, to more accurately indicate the role of an enzyme, both the object and the type of reaction are indicated in its name, for example, alcohol dehydrogenase is an enzyme that dehydrogenates alcohols.
For some enzymes discovered quite a long time ago, the historical name (without the ending -aza) has been preserved, for example, pepsin (pepsis, Greek. digestion) and trypsin (thrypsis Greek. liquefaction), these enzymes break down proteins.
For systematization, enzymes are combined into large classes, the classification is based on the type of reaction, the classes are named according to the general principle - the name of the reaction and the ending - aza. Some of these classes are listed below.
Oxidoreductase are enzymes that catalyze redox reactions. The dehydrogenases included in this class carry out proton transfer, for example, alcohol dehydrogenase (ADH) oxidizes alcohols to aldehydes, the subsequent oxidation of aldehydes to carboxylic acids is catalyzed by aldehyde dehydrogenases (ALDH). Both processes occur in the body during the processing of ethanol into acetic acid (Fig. 18).
Rice. eighteen TWO-STAGE OXIDATION OF ETHANOL to acetic acid
It is not ethanol that has a narcotic effect, but the intermediate product acetaldehyde, the lower the activity of the ALDH enzyme, the slower the second stage passes - the oxidation of acetaldehyde to acetic acid, and the longer and stronger the intoxicating effect from ingestion of ethanol. The analysis showed that more than 80% of the representatives of the yellow race have a relatively low activity of ALDH and therefore a markedly more severe alcohol tolerance. The reason for this innate reduced activity of ALDH is that part of the glutamic acid residues in the “attenuated” ALDH molecule is replaced by lysine fragments (Table 1).
Transferases- enzymes that catalyze the transfer of functional groups, for example, transiminase catalyzes the transfer of an amino group.
Hydrolases are enzymes that catalyze hydrolysis. The previously mentioned trypsin and pepsin hydrolyze peptide bonds, and lipases cleave the ester bond in fats:
–RC(O)OR 1 + H 2 O → –RC(O)OH + HOR 1
Liase- enzymes that catalyze reactions that take place in a non-hydrolytic way, as a result of such reactions, a rupture occurs C-C connections, C-O, C-N and the formation of new bonds. The enzyme decarboxylase belongs to this class
Isomerases- enzymes that catalyze isomerization, for example, the conversion of maleic acid to fumaric acid (Fig. 19), this is an example of cis-trans isomerization (see ISOMERIA).
Rice. nineteen. ISOMERIZATION OF MALEIC ACID into fumaric acid in the presence of the enzyme.
In the work of enzymes, the general principle is observed, according to which there is always a structural correspondence between the enzyme and the reagent of the accelerated reaction. According to the figurative expression of one of the founders of the doctrine of enzymes, E. Fisher, the reagent approaches the enzyme like a key to a lock. In this regard, each enzyme catalyzes a certain chemical reaction or a group of reactions of the same type. Sometimes an enzyme can act on a single compound, such as urease (uron Greek. - urine) catalyzes only the hydrolysis of urea:
(H 2 N) 2 C \u003d O + H 2 O \u003d CO 2 + 2NH 3
The finest selectivity is shown by enzymes that distinguish between optically active antipodes - left- and right-handed isomers. L-arginase acts only on levorotatory arginine and does not affect the dextrorotatory isomer. L-lactate dehydrogenase acts only on the levorotatory esters of lactic acid, the so-called lactates (lactis lat. milk), while D-lactate dehydrogenase only breaks down D-lactates.
Most of the enzymes act not on one, but on a group of related compounds, for example, trypsin "prefers" to cleave the peptide bonds formed by lysine and arginine (Table 1.)
The catalytic properties of some enzymes, such as hydrolases, are determined solely by the structure of the protein molecule itself, another class of enzymes - oxidoreductases (for example, alcohol dehydrogenase) can only be active in the presence of non-protein molecules associated with them - vitamins that activate Mg, Ca, Zn, Mn and fragments of nucleic acids (Fig. 20).
Rice. 20 ALCOHOLD DEHYDROGENASE MOLECULE
Transport proteins bind and transport various molecules or ions through cell membranes (both inside and outside the cell), as well as from one organ to another.
For example, hemoglobin binds oxygen as blood passes through the lungs and delivers it to various tissues of the body, where oxygen is released and then used to oxidize food components, this process serves as an energy source (sometimes they use the term "burning" food products in the body).
In addition to the protein part, hemoglobin contains a complex compound of iron with a cyclic porphyrin molecule (porphyros Greek. - purple), which determines the red color of the blood. It is this complex (Fig. 21, left) that plays the role of an oxygen carrier. In hemoglobin, the iron porphyrin complex is located inside the protein molecule and is retained by polar interactions, as well as by a coordination bond with nitrogen in histidine (Table 1), which is part of the protein. The O2 molecule, which is carried by hemoglobin, is attached via a coordination bond to the iron atom from the side opposite to that to which histidine is attached (Fig. 21, right).
Rice. 21 STRUCTURE OF THE IRON COMPLEX
The structure of the complex is shown on the right in the form of a three-dimensional model. The complex is held in the protein molecule by a coordination bond (dashed blue line) between the Fe atom and the N atom in histidine, which is part of the protein. The O 2 molecule, which is carried by hemoglobin, is coordinated (red dotted line) to the Fe atom from the opposite country of the planar complex.
Hemoglobin is one of the most studied proteins, it consists of a-helices connected by single chains and contains four iron complexes. Thus, hemoglobin is like a voluminous package for the transfer of four oxygen molecules at once. The form of hemoglobin corresponds to globular proteins (Fig. 22).
Rice. 22 GLOBULAR FORM OF HEMOGLOBIN
The main "advantage" of hemoglobin is that the addition of oxygen and its subsequent splitting off during transmission to various tissues and organs takes place quickly. Carbon monoxide, CO (carbon monoxide), binds to Fe in hemoglobin even faster, but, unlike O 2 , forms a complex that is difficult to break down. As a result, such hemoglobin is not able to bind O 2, which leads (when large amounts of carbon monoxide are inhaled) to the death of the body from suffocation.
The second function of hemoglobin is the transfer of exhaled CO 2, but not the iron atom, but the H 2 of the N-group of the protein is involved in the process of temporary binding of carbon dioxide.
The "performance" of proteins depends on their structure, for example, replacing the only amino acid residue of glutamic acid in the hemoglobin polypeptide chain with a valine residue (a rarely observed congenital anomaly) leads to a disease called sickle cell anemia.
There are also transport proteins that can bind fats, glucose, amino acids and carry them both inside and outside the cells.
Transport proteins of a special type do not carry the substances themselves, but act as a “transport regulator”, passing certain substances through the membrane (the outer wall of the cell). Such proteins are often called membrane proteins. They have the shape of a hollow cylinder and, being embedded in the membrane wall, ensure the movement of some polar molecules or ions into the cell. An example of a membrane protein is porin (Fig. 23).
Rice. 23 PORIN PROTEIN
Food and storage proteins, as the name implies, serve as sources of internal nutrition, more often for the embryos of plants and animals, as well as in the early stages of development of young organisms. Dietary proteins include albumin (Fig. 10) - the main component of egg white, as well as casein - the main protein of milk. Under the action of the enzyme pepsin, casein curdles in the stomach, which ensures its retention in the digestive tract and efficient absorption. Casein contains fragments of all the amino acids needed by the body.
In ferritin (Fig. 12), which is contained in the tissues of animals, iron ions are stored.
Myoglobin is also a storage protein, which resembles hemoglobin in composition and structure. Myoglobin is concentrated mainly in the muscles, its main role is the storage of oxygen, which hemoglobin gives it. It is rapidly saturated with oxygen (much faster than hemoglobin), and then gradually transfers it to various tissues.
Structural proteins perform a protective function (skin) or support - they hold the body together and give it strength (cartilage and tendons). Their main component is the fibrillar protein collagen (Fig. 11), the most common protein of the animal world, in the body of mammals, it accounts for almost 30% of the total mass of proteins. Collagen has a high tensile strength (the strength of the skin is known), but due to the low content of cross-links in skin collagen, animal skins are not very suitable in their raw form for the manufacture of various products. To reduce the swelling of the skin in water, shrinkage during drying, as well as to increase the strength in the watered state and increase the elasticity in collagen, additional cross-links are created (Fig. 15a), this is the so-called tanning process of the skin.
In living organisms, collagen molecules that have arisen in the process of growth and development of the organism are not updated and are not replaced by newly synthesized ones. As the body ages, the number of cross-links in collagen increases, which leads to a decrease in its elasticity, and since renewal does not occur, age-related changes appear - an increase in the fragility of cartilage and tendons, the appearance of wrinkles on the skin.
Articular ligaments contain elastin, a structural protein that easily stretches in two dimensions. The resilin protein, which is located at the points of hinge attachment of the wings in some insects, has the greatest elasticity.
Horn formations - hair, nails, feathers, consisting mainly of keratin protein (Fig. 24). Its main difference is the noticeable content of cysteine residues, which form disulfide bridges, which gives high elasticity (the ability to restore its original shape after deformation) to hair, as well as woolen fabrics.
Rice. 24. FRAGMENT OF FIBRILLAR PROTEIN KERATIN
For an irreversible change in the shape of a keratin object, you must first destroy the disulfide bridges with the help of a reducing agent, give new form, and then re-create disulfide bridges with the help of an oxidizing agent (Fig. 16), this is how, for example, perming hair is done.
With an increase in the content of cysteine residues in keratin and, accordingly, an increase in the number of disulfide bridges, the ability to deform disappears, but high strength appears at the same time (up to 18% of cysteine fragments are contained in the horns of ungulates and turtle shells). Mammals have up to 30 different types of keratin.
The keratin-related fibrillar protein fibroin secreted by silkworm caterpillars during cocoon curling, as well as by spiders during web weaving, contains only β-structures connected by single chains (Fig. 11). Unlike keratin, fibroin does not have transverse disulfide bridges, it has a very strong tensile strength (strength per unit cross-section of some web samples is higher than that of steel cables). Due to the absence of cross-links, fibroin is inelastic (it is known that woolen fabrics are almost indelible, and silk fabrics are easily wrinkled).
regulatory proteins.
Regulatory proteins, more commonly referred to as hormones, are involved in various physiological processes. For example, the hormone insulin (Fig. 25) consists of two α-chains connected by disulfide bridges. Insulin regulates metabolic processes involving glucose, its absence leads to diabetes.
Rice. 25 PROTEIN INSULIN
The pituitary gland of the brain synthesizes a hormone that regulates the growth of the body. There are regulatory proteins that control the biosynthesis of various enzymes in the body.
Contractile and motor proteins give the body the ability to contract, change shape and move, primarily we are talking about muscles. 40% of the mass of all proteins contained in the muscles is myosin (mys, myos, Greek. - muscle). Its molecule contains both a fibrillar and a globular part (Fig. 26)
Rice. 26 MYOSIN MOLECULE
Such molecules combine into large aggregates containing 300–400 molecules.
When the concentration of calcium ions changes in the space surrounding muscle fibers, a reversible change in the conformation of molecules occurs - a change in the shape of the chain due to the rotation of individual fragments around valence bonds. This leads to muscle contraction and relaxation, the signal to change the concentration of calcium ions comes from the nerve endings in the muscle fibers. Artificial muscle contraction can be caused by the action of electrical impulses, leading to a sharp change in the concentration of calcium ions, this is the basis for stimulating the heart muscle to restore the work of the heart.
Protective proteins allow you to protect the body from the invasion of attacking bacteria, viruses and from the penetration of foreign proteins (the generalized name of foreign bodies is antigens). The role of protective proteins is performed by immunoglobulins (their other name is antibodies), they recognize antigens that have penetrated the body and firmly bind to them. In the body of mammals, including humans, there are five classes of immunoglobulins: M, G, A, D and E, their structure, as the name implies, is globular, in addition, they are all built in a similar way. The molecular organization of antibodies is shown below using class G immunoglobulin as an example (Fig. 27). The molecule contains four polypeptide chains connected by three S-S disulfide bridges (in Fig. 27 they are shown with thickened valence bonds and large S symbols), in addition, each polymer chain contains intrachain disulfide bridges. Two large polymer chains (highlighted in blue) contain 400–600 amino acid residues. The other two chains (highlighted in green) are almost half as long, containing approximately 220 amino acid residues. All four chains are located in such a way that the terminal H 2 N-groups are directed in one direction.
Rice. 27 SCHEMATIC DRAWING OF THE STRUCTURE OF IMMUNOGLOBULIN
After the body comes into contact with a foreign protein (antigen), the cells of the immune system begin to produce immunoglobulins (antibodies), which accumulate in the blood serum. At the first stage, the main work is done by chain sections containing terminal H 2 N (in Fig. 27, the corresponding sections are marked in light blue and light green). These are antigen capture sites. In the process of immunoglobulin synthesis, these sites are formed in such a way that their structure and configuration correspond as much as possible to the structure of the approaching antigen (like a key to a lock, like enzymes, but the tasks in this case are different). Thus, for each antigen, a strictly individual antibody is created as an immune response. Not a single known protein can change its structure so “plastically” depending on external factors, in addition to immunoglobulins. Enzymes solve the problem of structural conformity to the reagent in a different way - with the help of a gigantic set of various enzymes for all possible cases, and immunoglobulins each time rebuild the "working tool". Moreover, the hinge region of the immunoglobulin (Fig. 27) provides the two capture regions with some independent mobility, as a result, the immunoglobulin molecule can immediately “find” the two most convenient regions for capture in the antigen in order to securely fix it, this resembles the actions of a crustacean creature.
Next, a chain of successive reactions of the body's immune system is turned on, immunoglobulins of other classes are connected, as a result, the foreign protein is deactivated, and then the antigen (foreign microorganism or toxin) is destroyed and removed.
After contact with the antigen, the maximum concentration of immunoglobulin is reached (depending on the nature of the antigen and the individual characteristics of the organism itself) within a few hours (sometimes several days). The body retains the memory of such contact, and when attacked again with the same antigen, immunoglobulins accumulate in the blood serum much faster and in greater quantities - acquired immunity occurs.
The above classification of proteins is somewhat arbitrary, for example, the thrombin protein, mentioned among protective proteins, is essentially an enzyme that catalyzes the hydrolysis of peptide bonds, that is, it belongs to the class of proteases.
Protective proteins are often referred to as snake venom proteins and the toxic proteins of some plants, since their task is to protect the body from damage.
There are proteins whose functions are so unique that it makes it difficult to classify them. For example, the protein monellin, found in an African plant, is very sweet tasting and has been the subject of research as a non-toxic substance that can be used in place of sugar to prevent obesity. The blood plasma of some Antarctic fish contains proteins with antifreeze properties that keep the blood of these fish from freezing.
Artificial synthesis of proteins.
The condensation of amino acids leading to a polypeptide chain is a well-studied process. It is possible to carry out, for example, the condensation of any one amino acid or a mixture of acids and obtain, respectively, a polymer containing the same units, or different units, alternating in random order. Such polymers bear little resemblance to natural polypeptides and do not possess biological activity. The main task is to connect amino acids in a strictly defined, pre-planned order in order to reproduce the sequence of amino acid residues in natural proteins. The American scientist Robert Merrifield proposed an original method that made it possible to solve such a problem. The essence of the method is that the first amino acid is attached to an insoluble polymer gel that contains reactive groups that can combine with –COOH – groups of the amino acid. Cross-linked polystyrene with chloromethyl groups introduced into it was taken as such a polymeric substrate. So that the amino acid taken for the reaction does not react with itself and so that it does not join the H 2 N-group to the substrate, the amino group of this acid is pre-blocked with a bulky substituent [(C 4 H 9) 3] 3 OS (O) -group. After the amino acid has attached to the polymeric support, the blocking group is removed and another amino acid is introduced into the reaction mixture, in which the H 2 N group is also previously blocked. In such a system, only the interaction of the H 2 N-group of the first amino acid and the –COOH group of the second acid is possible, which is carried out in the presence of catalysts (phosphonium salts). Then the whole scheme is repeated, introducing the third amino acid (Fig. 28).
Rice. 28. SYNTHESIS SCHEME OF POLYPEPTIDE CHAINS
In the last step, the resulting polypeptide chains are separated from the polystyrene support. Now the whole process is automated, there are automatic peptide synthesizers that operate according to the described scheme. Many peptides used in medicine and agriculture have been synthesized by this method. It was also possible to obtain improved analogues of natural peptides with selective and enhanced action. Some small proteins have been synthesized, such as the hormone insulin and some enzymes.
There are also methods of protein synthesis that replicate natural processes: fragments of nucleic acids are synthesized that are configured to produce certain proteins, then these fragments are inserted into a living organism (for example, into a bacterium), after which the body begins to produce the desired protein. In this way, significant amounts of hard-to-reach proteins and peptides, as well as their analogues, are now obtained.
Proteins as food sources.
Proteins in a living organism are constantly broken down into their original amino acids (with the indispensable participation of enzymes), some amino acids pass into others, then proteins are synthesized again (also with the participation of enzymes), i.e. the body is constantly renewing itself. Some proteins (collagen of the skin, hair) are not renewed, the body continuously loses them and instead synthesizes new ones. Proteins as food sources perform two main functions: they supply the body with building material for the synthesis of new protein molecules and, in addition, supply the body with energy (sources of calories).
Carnivorous mammals (including humans) get the necessary proteins from plant and animal foods. None of the proteins obtained from food is integrated into the body in an unchanged form. In the digestive tract, all absorbed proteins are broken down to amino acids, and proteins necessary for a particular organism are already built from them, while the remaining 12 can be synthesized from 8 essential acids (Table 1) in the body if they are not supplied in sufficient quantities with food, but essential acids must be supplied with food without fail. Sulfur atoms in cysteine are obtained by the body with the essential amino acid methionine. Part of the proteins breaks down, releasing the energy necessary to maintain life, and the nitrogen contained in them is excreted from the body with urine. Usually the human body loses 25–30 g of protein per day, so protein foods must always be present in the right amount. The minimum daily requirement for protein is 37 g for men and 29 g for women, but the recommended intake is almost twice as high. When evaluating foods, it is important to consider protein quality. In the absence or low content of essential amino acids, the protein is considered of low value, so such proteins should be consumed in greater quantities. So, the proteins of legumes contain little methionine, and the proteins of wheat and corn are low in lysine (both amino acids are essential). Animal proteins (excluding collagens) are classified as complete foods. A complete set of all essential acids contains milk casein, as well as cottage cheese and cheese prepared from it, so a vegetarian diet, if it is very strict, i.e. “dairy-free”, requires increased consumption of legumes, nuts and mushrooms to supply the body with essential amino acids in the right amount.
Synthetic amino acids and proteins are also used as food products, adding them to feed, which contain essential amino acids in small quantities. There are bacteria that can process and assimilate oil hydrocarbons, in this case, for the full synthesis of proteins, they need to be fed with nitrogen-containing compounds (ammonia or nitrates). The protein obtained in this way is used as feed for livestock and poultry. A set of enzymes, carbohydrases, are often added to animal feed, which catalyze the hydrolysis of carbohydrate food components that are difficult to decompose (cell walls of cereal crops), as a result of which plant foods are more fully absorbed.
Mikhail Levitsky
PROTEINS (Article 2)
(proteins), a class of complex nitrogen-containing compounds, the most characteristic and important (along with nucleic acids) components of living matter. Proteins perform many and varied functions. Most proteins are enzymes that catalyze chemical reactions. Many hormones that regulate physiological processes are also proteins. Structural proteins such as collagen and keratin are the main components bone tissue, hair and nails. The contractile proteins of muscles have the ability to change their length, using chemical energy to perform mechanical work. Proteins are antibodies that bind and neutralize toxic substances. Some proteins that can respond to external influences (light, smell) serve as receptors in the sense organs that perceive irritation. Many proteins located inside the cell and on the cell membrane perform regulatory functions.
In the first half of the 19th century many chemists, and among them primarily J. von Liebig, gradually came to the conclusion that proteins are a special class of nitrogenous compounds. The name "proteins" (from the Greek protos - the first) was proposed in 1840 by the Dutch chemist G. Mulder.
PHYSICAL PROPERTIES
Proteins in the solid state white color, and are colorless in solution, unless they carry some chromophore (colored) group, such as hemoglobin. The solubility in water of different proteins varies greatly. It also varies with pH and with the concentration of salts in the solution, so that one can choose the conditions under which one protein will selectively precipitate in the presence of other proteins. This "salting out" method is widely used to isolate and purify proteins. The purified protein often precipitates out of solution as crystals.
In comparison with other compounds, the molecular weight of proteins is very large - from several thousand to many millions of daltons. Therefore, during ultracentrifugation, proteins are precipitated, and, moreover, at different rates. Due to the presence of positively and negatively charged groups in protein molecules, they move at different speeds in an electric field. This is the basis of electrophoresis, a method used to isolate individual proteins from complex mixtures. Purification of proteins is also carried out by chromatography.
CHEMICAL PROPERTIES
Structure.
Proteins are polymers, i.e. molecules built like chains from repeating monomer units, or subunits, whose role is played by alpha-amino acids. General formula of amino acids
where R is a hydrogen atom or some organic group.
A protein molecule (polypeptide chain) may consist of only a relatively small number of amino acids or several thousand monomer units. The connection of amino acids in the chain is possible because each of them has two different chemical groups: a basic amino group, NH2, and an acidic carboxyl group, COOH. Both of these groups are attached to the a carbon atom. The carboxyl group of one amino acid can form an amide (peptide) bond with the amino group of another amino acid:
After two amino acids have been connected in this way, the chain can be extended by adding a third to the second amino acid, and so on. As can be seen from the above equation, when a peptide bond is formed, a water molecule is released. In the presence of acids, alkalis or proteolytic enzymes, the reaction proceeds in the opposite direction: the polypeptide chain is cleaved into amino acids with the addition of water. This reaction is called hydrolysis. Hydrolysis proceeds spontaneously, and energy is required to combine amino acids into a polypeptide chain.
A carboxyl group and an amide group (or an imide group similar to it - in the case of the proline amino acid) are present in all amino acids, while the differences between amino acids are determined by the nature of that group, or "side chain", which is indicated above by the letter R. The role of the side chain can be played by one a hydrogen atom, like the amino acid glycine, and some bulky grouping, like histidine and tryptophan. Some side chains are chemically inert, while others are highly reactive.
Many thousands of different amino acids can be synthesized, and many different amino acids occur in nature, but only 20 types of amino acids are used for protein synthesis: alanine, arginine, asparagine, aspartic acid, valine, histidine, glycine, glutamine, glutamic acid, isoleucine, leucine, lysine , methionine, proline, serine, tyrosine, threonine, tryptophan, phenylalanine and cysteine (in proteins, cysteine may be present as a dimer - cystine). True, there are other amino acids in some proteins, in addition to the regularly occurring twenty, but they are formed as a result of modification of any of the twenty listed after it has been included in the protein.
optical activity.
All amino acids, with the exception of glycine, have four different groups attached to the α-carbon atom. In terms of geometry, four different groups can be attached in two ways, and accordingly there are two possible configurations, or two isomers, related to each other as an object to its mirror image, i.e. as left hand to the right. One configuration is called left, or left-handed (L), and the other right-handed, or right-handed (D), because the two such isomers differ in the direction of rotation of the plane of polarized light. Only L-amino acids occur in proteins (the exception is glycine; it can only be represented in one form, since two of its four groups are the same), and they all have optical activity (since there is only one isomer). D-amino acids are rare in nature; they are found in some antibiotics and the cell wall of bacteria.
The sequence of amino acids.
Amino acids in the polypeptide chain are not arranged randomly, but in a certain fixed order, and it is this order that determines the functions and properties of the protein. By varying the order of the 20 types of amino acids, you can get a huge number of different proteins, just like you can make up many different texts from the letters of the alphabet.
In the past, determining the amino acid sequence of a protein often took several years. Direct determination is still a rather laborious task, although devices have been created that allow it to be carried out automatically. It is usually easier to determine the nucleotide sequence of the corresponding gene and derive the amino acid sequence of the protein from it. To date, the amino acid sequences of many hundreds of proteins have already been determined. The functions of decoded proteins are usually known, and this helps to imagine the possible functions of similar proteins formed, for example, in malignant neoplasms.
Complex proteins.
Proteins consisting of only amino acids are called simple. Often, however, a metal atom or some other element is attached to the polypeptide chain. chemical compound, which is not an amino acid. Such proteins are called complex. An example is hemoglobin: it contains iron porphyrin, which gives it its red color and allows it to act as an oxygen carrier.
The names of most complex proteins contain an indication of the nature of the attached groups: sugars are present in glycoproteins, fats in lipoproteins. If the catalytic activity of the enzyme depends on the attached group, then it is called a prosthetic group. Often, some vitamin plays the role of a prosthetic group or is part of it. Vitamin A, for example, attached to one of the proteins of the retina, determines its sensitivity to light.
Tertiary structure.
What is important is not so much the amino acid sequence of the protein (primary structure), but the way it is laid in space. Along the entire length of the polypeptide chain, hydrogen ions form regular hydrogen bonds, which give it the shape of a spiral or layer (secondary structure). From the combination of such helices and layers, a compact form of the next order arises - the tertiary structure of the protein. Around the bonds that hold the monomeric links of the chain, rotations through small angles are possible. Therefore, with pure geometric point From the point of view, the number of possible configurations for any polypeptide chain is infinitely large. In reality, each protein normally exists in only one configuration, determined by its amino acid sequence. This structure is not rigid, it seems to "breathe" - it oscillates around a certain average configuration. The chain is folded into a configuration in which the free energy (the ability to do work) is minimal, just as a released spring is compressed only to a state corresponding to a minimum of free energy. Often, one part of the chain is rigidly linked to the other by disulfide (–S–S–) bonds between two cysteine residues. This is partly why cysteine among amino acids plays a particularly important role.
The complexity of the structure of proteins is so great that it is not yet possible to calculate the tertiary structure of a protein, even if its amino acid sequence is known. But if it is possible to obtain protein crystals, then its tertiary structure can be determined by X-ray diffraction.
In structural, contractile, and some other proteins, the chains are elongated and several slightly folded chains lying side by side form fibrils; fibrils, in turn, fold into larger formations - fibers. However, most proteins in solution are globular: the chains are coiled in a globule, like yarn in a ball. Free energy in this configuration is minimal, since the hydrophobic (“water-repelling”) amino acids are hidden inside the globule, while the hydrophilic (“water-attracting”) amino acids are on its surface.
Many proteins are complexes of several polypeptide chains. This structure is called the quaternary structure of the protein. The hemoglobin molecule, for example, is made up of four subunits, each of which is a globular protein.
Structural proteins, due to their linear configuration, form fibers in which the tensile strength is very high, while the globular configuration allows proteins to enter into specific interactions with other compounds. On the surface of the globule, with the correct laying of chains, cavities of a certain shape appear, in which reactive chemical groups are located. If this protein is an enzyme, then another, usually smaller, molecule of some substance enters such a cavity, just as a key enters a lock; in this case, the configuration of the electron cloud of the molecule changes under the influence of chemical groups located in the cavity, and this forces it to react in a certain way. In this way, the enzyme catalyzes the reaction. Antibody molecules also have cavities in which various foreign substances bind and are thereby rendered harmless. The "key and lock" model, which explains the interaction of proteins with other compounds, makes it possible to understand the specificity of enzymes and antibodies, i.e. their ability to react only with certain compounds.
Proteins in different types of organisms.
Proteins that perform the same function in different plant and animal species and therefore bear the same name also have a similar configuration. They, however, differ somewhat in their amino acid sequence. As species diverge from a common ancestor, some amino acids in certain positions are replaced by mutations with others. Harmful mutations that cause hereditary diseases are discarded natural selection, but useful or at least neutral ones can persist. The closer two biological species are to each other, the less differences are found in their proteins.
Some proteins change relatively quickly, others are quite conservative. The latter include, for example, cytochrome c, a respiratory enzyme found in most living organisms. In humans and chimpanzees, its amino acid sequences are identical, while in cytochrome c of wheat, only 38% of the amino acids turned out to be different. Even when comparing humans and bacteria, the similarities of cytochromes with (the differences here affect 65% of amino acids) can still be seen, although the common ancestor of bacteria and humans lived on Earth about two billion years ago. Nowadays, comparison of amino acid sequences is often used to build a phylogenetic (genealogical) tree that reflects the evolutionary relationships between different organisms.
Denaturation.
The synthesized protein molecule, folding, acquires its own configuration. This configuration, however, can be destroyed by heating, by changing the pH, by the action of organic solvents, and even by simply agitating the solution until bubbles appear on its surface. A protein altered in this way is called denatured; it loses its biological activity and usually becomes insoluble. Well-known examples of denatured protein are boiled eggs or whipped cream. Small proteins, containing only about a hundred amino acids, are able to renature, i.e. reacquire the original configuration. But most of the proteins are simply transformed into a mass of tangled polypeptide chains and do not restore their previous configuration.
One of the main difficulties in isolating active proteins is their extreme sensitivity to denaturation. This property of proteins finds useful application in the preservation of food products: high temperature irreversibly denatures the enzymes of microorganisms, and the microorganisms die.
PROTEIN SYNTHESIS
For protein synthesis, a living organism must have a system of enzymes capable of attaching one amino acid to another. A source of information is also needed that would determine which amino acids should be connected. Since there are thousands of types of proteins in the body, and each of them consists of an average of several hundred amino acids, the information required must be truly enormous. It is stored (similar to how a record is stored on a magnetic tape) in the nucleic acid molecules that make up genes.
Enzyme activation.
A polypeptide chain synthesized from amino acids is not always a protein in its final form. Many enzymes are first synthesized as inactive precursors and become active only after another enzyme removes a few amino acids from one end of the chain. Some of the digestive enzymes, such as trypsin, are synthesized in this inactive form; these enzymes are activated in the digestive tract as a result of the removal of the terminal fragment of the chain. The hormone insulin, whose molecule in its active form consists of two short chains, is synthesized in the form of a single chain, the so-called. proinsulin. Then the middle part of this chain is removed, and the remaining fragments bind to each other, forming the active hormone molecule. Complex proteins are formed only after a certain chemical group is attached to the protein, and this attachment often also requires an enzyme.
Metabolic circulation.
After feeding an animal with amino acids labeled with radioactive isotopes of carbon, nitrogen or hydrogen, the label is quickly incorporated into its proteins. If labeled amino acids cease to enter the body, then the amount of label in proteins begins to decrease. These experiments show that the resulting proteins are not stored in the body until the end of life. All of them, with a few exceptions, are in a dynamic state, constantly decomposing to amino acids, and then re-synthesized.
Some proteins break down when cells die and are destroyed. This happens all the time, for example, with red blood cells and epithelial cells lining the inner surface of the intestine. In addition, the breakdown and resynthesis of proteins also occur in living cells. Oddly enough, less is known about the breakdown of proteins than about their synthesis. What is clear, however, is that proteolytic enzymes are involved in the breakdown, similar to those that break down proteins into amino acids in the digestive tract.
The half-life of different proteins is different - from several hours to many months. The only exception is collagen molecules. Once formed, they remain stable and are not renewed or replaced. Over time, however, some of their properties, in particular elasticity, change, and since they are not renewed, certain age-related changes, such as the appearance of wrinkles on the skin, are the result of this.
synthetic proteins.
Chemists have long since learned how to polymerize amino acids, but the amino acids are combined randomly, so that the products of such polymerization bear little resemblance to natural ones. True, it is possible to combine amino acids in a given order, which makes it possible to obtain some biologically active proteins, in particular insulin. The process is quite complicated, and in this way it is possible to obtain only those proteins whose molecules contain about a hundred amino acids. It is preferable instead to synthesize or isolate the nucleotide sequence of a gene corresponding to the desired amino acid sequence, and then introduce this gene into a bacterium, which will produce by replication a large amount of the desired product. This method, however, also has its drawbacks.
PROTEINS AND NUTRITION
When proteins in the body are broken down into amino acids, these amino acids can be reused for protein synthesis. At the same time, the amino acids themselves are subject to decay, so that they are not fully utilized. It is also clear that during growth, pregnancy, and wound healing, protein synthesis must exceed degradation. The body continuously loses some proteins; these are the proteins of hair, nails and the surface layer of the skin. Therefore, for the synthesis of proteins, each organism must receive amino acids from food.
Sources of amino acids.
Green plants synthesize all 20 amino acids found in proteins from CO2, water and ammonia or nitrates. Many bacteria are also able to synthesize amino acids in the presence of sugar (or some equivalent) and fixed nitrogen, but sugar is ultimately supplied by green plants. In animals, the ability to synthesize amino acids is limited; they obtain amino acids by eating green plants or other animals. In the digestive tract, the absorbed proteins are broken down into amino acids, the latter are absorbed, and the proteins characteristic of the given organism are built from them. None of the absorbed protein is incorporated into body structures as such. The only exception is that in many mammals, part of the maternal antibodies can pass intact through the placenta into the fetal circulation, and through the mother's milk (especially in ruminants) be transferred to the newborn immediately after birth.
Need for proteins.
It is clear that in order to maintain life, the body must receive a certain amount of protein from food. However, the size of this need depends on a number of factors. The body needs food both as a source of energy (calories) and as a material for building its structures. In the first place is the need for energy. This means that when there are few carbohydrates and fats in the diet, dietary proteins are used not for the synthesis of their own proteins, but as a source of calories. With prolonged fasting, even your own proteins are spent to meet energy needs. If there are enough carbohydrates in the diet, then protein intake can be reduced.
nitrogen balance.
On average approx. 16% of the total protein mass is nitrogen. When the amino acids that make up proteins are broken down, the nitrogen contained in them is excreted from the body in the urine and (to a lesser extent) in the feces in the form of various nitrogenous compounds. Therefore, it is convenient to use such an indicator as nitrogen balance to assess the quality of protein nutrition, i.e. the difference (in grams) between the amount of nitrogen taken into the body and the amount of nitrogen excreted per day. With normal nutrition in an adult, these amounts are equal. In a growing organism, the amount of excreted nitrogen is less than the amount of incoming, i.e. the balance is positive. With a lack of protein in the diet, the balance is negative. If there are enough calories in the diet, but the proteins are completely absent in it, the body saves proteins. At the same time, protein metabolism slows down, and the re-utilization of amino acids in protein synthesis proceeds as efficiently as possible. However, losses are inevitable, and nitrogenous compounds are still excreted in the urine and partly in the feces. The amount of nitrogen excreted from the body per day during protein starvation can serve as a measure of the daily lack of protein. It is natural to assume that by introducing into the diet an amount of protein equivalent to this deficiency, it is possible to restore the nitrogen balance. However, it is not. Having received this amount of protein, the body begins to use amino acids less efficiently, so some additional protein is required to restore the nitrogen balance.
If the amount of protein in the diet exceeds what is necessary to maintain nitrogen balance, then there seems to be no harm from this. Excess amino acids are simply used as a source of energy. A particularly striking example is the Eskimo, who consume little carbohydrate and about ten times more protein than is required to maintain nitrogen balance. In most cases, however, using protein as an energy source is not beneficial, since you can get many more calories from a given amount of carbohydrates than from the same amount of protein. In poor countries, the population receives the necessary calories from carbohydrates and consumes a minimum amount of protein.
If the body receives the required number of calories in the form of non-protein foods, then the minimum amount of protein that maintains the nitrogen balance is approx. 30 g per day. Approximately as much protein is contained in four slices of bread or 0.5 liters of milk. A slightly larger amount is usually considered optimal; recommended from 50 to 70 g.
Essential amino acids.
Until now, protein has been considered as a whole. Meanwhile, in order for protein synthesis to take place, all the necessary amino acids must be present in the body. Some of the amino acids the body of the animal itself is able to synthesize. They are called interchangeable, since they do not have to be present in the diet, it is only important that, in general, the intake of protein as a source of nitrogen is sufficient; then, with a shortage of non-essential amino acids, the body can synthesize them at the expense of those that are present in excess. The remaining "essential" amino acids cannot be synthesized and must be ingested with food. Essential for humans are valine, leucine, isoleucine, threonine, methionine, phenylalanine, tryptophan, histidine, lysine, and arginine. (Although arginine can be synthesized in the body, it is considered an essential amino acid because newborns and growing children produce insufficient amounts of it. On the other hand, for a person of mature age, the intake of some of these amino acids from food may become optional.)
This list of essential amino acids is approximately the same in other vertebrates and even in insects. The nutritional value of proteins is usually determined by feeding them to growing rats and monitoring the weight gain of the animals.
The nutritional value of proteins.
The nutritional value of a protein is determined by the essential amino acid that is most deficient. Let's illustrate this with an example. The proteins of our body contain an average of approx. 2% tryptophan (by weight). Let's say that the diet includes 10 g of protein containing 1% tryptophan, and that there are enough other essential amino acids in it. In our case, 10 g of this defective protein is essentially equivalent to 5 g of a complete one; the remaining 5 g can only serve as a source of energy. Note that since amino acids are practically not stored in the body, and in order for protein synthesis to take place, all amino acids must be present simultaneously, the effect of the intake of essential amino acids can be detected only if all of them enter the body at the same time.
The average composition of most animal proteins is close to the average composition of proteins in the human body, so we are unlikely to face amino acid deficiency if our diet is rich in foods such as meat, eggs, milk and cheese. However, there are proteins, such as gelatin (a product of collagen denaturation), which contain very few essential amino acids. Vegetable proteins, although they are better than gelatin in this sense, are also poor in essential amino acids; especially little in them lysine and tryptophan. However, a purely vegetarian diet is by no means unhealthy, unless it consumes a slightly larger amount of vegetable proteins, sufficient to provide the body with essential amino acids. Most protein is found in plants in the seeds, especially in the seeds of wheat and various legumes. Young shoots, such as asparagus, are also rich in protein.
Synthetic proteins in the diet.
By adding small amounts of synthetic essential amino acids or proteins rich in them to incomplete proteins, such as corn proteins, one can significantly increase the nutritional value of the latter, i.e. thereby increasing the amount of protein consumed. Another possibility is to grow bacteria or yeasts on petroleum hydrocarbons with the addition of nitrates or ammonia as a source of nitrogen. The microbial protein obtained in this way can serve as feed for poultry or livestock, or can be directly consumed by humans. The third, widely used, method uses the physiology of ruminants. In ruminants, in the initial section of the stomach, the so-called. In the rumen, there are special forms of bacteria and protozoa that convert defective plant proteins into more complete microbial proteins, and these, in turn, after digestion and absorption, turn into animal proteins. Urea, a cheap synthetic nitrogen-containing compound, can be added to livestock feed. Microorganisms living in the rumen use urea nitrogen to convert carbohydrates (of which there is much more in the feed) into protein. About a third of all nitrogen in livestock feed can come in the form of urea, which in essence means, to a certain extent, chemical protein synthesis.