As already indicated, another category of polymer production reactions by nature are stepwise processes, which include polycondensation and stepwise polymerization. In these reactions, growing chains of polymers after each act of addition are stable particles, the process of polymer formation proceeds in steps, and the molecular weight increases gradually.
During stepwise polymerization and polycondensation and during chain polymerization, different times are spent to obtain a high-molecular product, i.e., to complete the growth of the macromolecule chain. During polycondensation, for example, which occurs in a stepwise manner, the size of the molecule increases at a relatively low rate and first a dimer, trimer, tetramer, etc., is formed from monomers to a polymer. In chain polymerization, molecules with high molecular weight are formed almost immediately after the start of the reaction. In the latter case, at various stages of the process, only monomer and polymer are always present in the reaction mixture and there are no molecules of intermediate sizes. As the reaction time increases, only the number of polymer molecules increases. The molecular weight of the polymer does not depend on the degree of completion of the reaction, which only affects the yield of the polymer. During polycondensation, the formation of a polymer occurs at a stage very high degree completion of the reaction (more than 98%), both the yield and the molecular weight of the polymer depend on the duration of the reaction.
The original molecules and those obtained as a result of polycondensation are stable and can be isolated. However, they contain reactive groups at the ends and can participate in further condensation reactions with each other or with other monomers. This is used in industry to obtain oligomers and synthesize various polymers from them, including those with a spatially cross-linked structure.
Polycondensation, in which only bifunctional molecules participate, leads to the formation of linear polymer molecules and is called linear.
For example, the formation of polyamide:
In this case, the same principle of constructing macromolecules can be implemented both in the reaction of two different bifunctional monomers, each of which contains only one type of functional groups (a), and from one monomer containing both types of functional groups (b). Case (a) corresponds to copolycondensation, case (b) to homopolycondensation.
A polycondensation process involving molecules with three or a large number functional groups, leads to the formation of branched or three-dimensional (network, cross-linked) structures and is called three-dimensional
polycondensation. For example, the formation of phenol-formaldehyde resins:


A similar process is the polycondensation of glycerol and phthalic acid (glyphthalic resins), silanetriols, etc.
Polycondensation is an equilibrium process, i.e. condensation products can react with by-products of low molecular weight substances to form the original compounds.
Thus, the equilibrium of the reaction must be shifted to the right as a result of removing the low molecular weight product (ab) from the reaction zone (for example, by distillation, vacuum). Due to the staged nature of the polycondensation reaction (monomer + monomer ® dimer; dimer + monomer ® trimer; dimer + dimer ® tetramer; trimer + dimer ® pentamer, etc.), the molecular weight of the products continuously increases and the monomer disappears long before the formation of a polymer with a molecular weight of more 5000-10000. In most polycondensation reactions, no more than 1% of the original monomer remains at the time the polymer is formed.
In the linear polycondensation of two monomers, in order to obtain the highest possible molecular weight of the polymer, it is necessary to maintain equality in the concentrations of the starting components. Increasing the concentration of one of them sharply reduces the degree of polycondensation, since the functional groups of the excess monomer act as inhibitors and stop the reaction in the early stages, i.e., before the formation of the polymer.
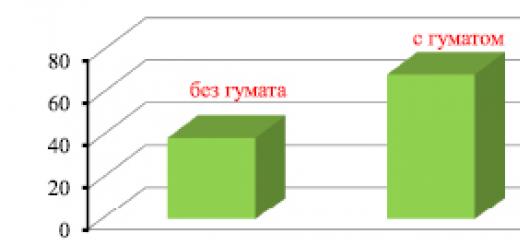
When carrying out polycondensation, it is very important to know the dependence of its speed on various factors, the dependence of the degree of polycondensation on the depth of monomer conversion, the ratio of monomers in the mixture, and other reasons for the cessation of growth in the molecular weight of the polymer (it is usually much less than during polymerization). The dependence of the limiting degree of polycondensation on the concentration of the released low-molecular compound and the equilibrium constant is characterized by the polycondensation equilibrium equation:
where P is the degree of polycondensation; k is the equilibrium constant; na- mole fraction low molecular weight substance released during the reaction. The dependence of the degree of polycondensation on the depth of monomer conversion is expressed by the curve shown in Fig. 10. Here you can see that the polymer is formed only after the bulk of the monomer has been consumed.

Three-dimensional polycondensation differs from linear polycondensation in the higher rate constant of the direct reaction due mainly to the transition of the system into a gel after the start of the reaction. The branched structure of the polymer is formed by the reaction of bifunctional and trifunctional molecules with each other. A trifunctional molecule gives rise to branching, the chains branch one after another and ultimately form an infinite network. For example, the condensation of trihydric alcohol - glycerol and dibasic phthalic acid. The higher the functionality of the monomers, the lower the degree of completion of the reaction, gelation occurs. Due to the formation of a low-mobility branched or network structure, the requirements for maintaining equal concentrations of functional groups and removing low molecular weight polycondensation products are not as stringent as for linear polycondensation.
Stepwise (or migration) polymerization is similar in its basic principles and structure of the resulting polymer to linear polycondensation. The addition of each subsequent monomer to the growing chain, which is also a stable particle, is carried out by the movement (migration) of hydrogen. This process occurs during the synthesis of polyurethanes from isocyanates and glycols:


etc. until a polymer is formed
The difference between stepwise polymerization and polycondensation is that there is no release of a low-molecular-weight reaction byproduct. If you replace glycol polyhydric alcohol(glycerol, pentaerythritol, etc.) or diisocyanate triisocyanate, then spatial polymers are obtained; the reaction of their formation is similar to three-dimensional polycondensation.
Polymerization due to ring opening of monomer molecules also often occurs via a stepwise reaction mechanism (for example, the polymerization of e-caprolactam). This process is activated by small amounts of water, acid, base:

As can be seen, the activator attaches only to the first molecule of the monomer, and during the growth of the chain, functional groups move to the end of the chain, i.e., migration polymerization occurs.
Cyclic monomers can also polymerize by an ionic mechanism (eg, ethylene oxide, trioxane, e-caprolactam with sodium metal, propylene oxide). When a ring breaks, the same types of bonds are restored by connecting two, three, etc. broken rings into a chain.
Polymers – these are high molecular weight compounds (HMW). Monomers are low molecular weight substances from which polymers are obtained. The degree of polymerization (polycondensation) is the average number of structural units in a polymer molecule.
Polymerization - a reaction of combining molecules of monomer m, not accompanied by the release of by-products. Therefore, the elemental composition of the monomers and the resulting polymer is the same. Polymerization can be carried out by opening double and triple bonds of unsaturated compounds, as well as by opening various heterocycles. Depending on the nature of the active centers that initiate the chain process, radical and ionic polymerization are distinguished. The process follows a chain mechanism.
nCH2=CH2→(-CH-CH-)n, where n is the degree of polymerization of the molecules, indicating how many monomer units are included in its composition.
Classification of polymers :
If we take the qualitative composition of molecules as a basis, then all the substances under consideration can be divided into three groups.
Organic are those that contain atoms of carbon, hydrogen, sulfur, oxygen, phosphorus, and nitrogen. That is, those elements that are biogenic. There are a lot of examples: polyethylene, polyvinyl chloride, polypropylene, viscose, nylon, natural polymer - protein, nucleic acids, and so on.
Organic elements are those that contain some foreign inorganic and non-biogenic element. Most often it is silicon, aluminum or titanium. Examples of such macromolecules: organic glass, glass polymers, composite materials.
Inorganic - the chain is based on silicon atoms, not carbon. Radicals can also be part of side branches. They were discovered quite recently, in the middle of the 20th century. Used in medicine, construction, technology and other industries. Examples: silicone, cinnabar.
If we divide polymers by origin, we can distinguish three groups.
Natural polymers, the use of which has been widely carried out since ancient times. These are macromolecules for which man did not make any effort to create. They are products of reactions of nature itself. Examples: silk, wool, protein, nucleic acids, starch, cellulose, leather, cotton and others.
Artificial. These are macromolecules that are created by humans, but based on natural analogues. That is, the properties of an existing natural polymer are simply improved and changed. Examples: artificial rubber, rubber.
Synthetic polymers are those in which only humans are involved in their creation. There are no natural analogues for them. Scientists are developing methods for synthesizing new materials that would have improved technical characteristics. This is how synthetic polymer compounds of various kinds are born. Examples: polyethylene, polypropylene, viscose, acetate fiber, etc.
Polycondensation – the reaction of the formation of high-molecular compounds, proceeding by the substitution mechanism and usually accompanied by the release of low-molecular products, as a result of which the elementary composition of the polymer differs from the elemental composition of the original products.
Monomers containing two or more functional groups can enter into the polycondensation reaction. When these groups interact, the molecule of a low-molecular compound decomposes, with the formation new group, which binds the residues of reacting molecules.
Polycondensation - stepwise reaction, chain growth occurs as a result of the interaction of monomer molecules with each other, as well as intermediate products: oligomeric or polymer molecules, or the interaction of oligomeric and polymer molecules with each other. As a result, compounds are formed with the functionality of the original substance.
During the polymerization reaction, only polymers are obtained as a result. During polycondensation, the reaction product becomes polymers and low molecular weight substances.
Definition
In progress polymerization both identical and different molecules of monomers are connected sequentially, building one complex molecule polymer (high molecular weight substance) without the release and formation of by-products - low molecular weight compounds. Therefore, the output is a polymer with exactly the same elemental composition as the monomer.
In progress polycondensation molecules of one or more monomers, connecting with each other, form a polymer macromolecule and by-product release one or another low-molecular product (water, alcohol, hydrogen chloride or ammonia). Polycondensation underlies the biosynthesis of cellulose, nucleic acids and, of course, proteins.
Comparison
These two processes are similar in that at the beginning of the reaction the original monomer enters into the reaction. And then during polymerization in the reaction system at all stages of the current process there are increasing active chains, the original monomer and macromolecules that have completed growth. And in the process of polycondensation, the monomer, as a rule, is exhausted at the initial stages of the ongoing reaction, and subsequently only polymers (oligomers) remain in the system, interacting with one another.
For polymerization and polycondensation, the reactivity of the desired monomers and, of course, their structure are equally important. During polymerization, reactions that occur between increasing molecules usually end in chain termination.
And during polycondensation, the reactions occurring between increasing molecules are the main reactions of growth of polymer chains. Long chains are formed due to the interaction of oligomers. Polymerization occurs in three stages: initiation, chain growth and chain termination. In this case, the centers of growth of the polymer chain are cations, free radicals or anions. Functionality (the number of reaction centers in a molecule) influences the formation of three-dimensional, branched or linear macromolecules.
Conclusions website
- Polycondensation is characterized by the release of by-products - low molecular weight substances such as water or alcohol.
- During polymerization, only polymers become reaction products.
- The biosynthesis of cellulose, proteins and nucleic acids is possible due to the polycondensation reaction.
Polymerization reactions
Polymerization is the reaction of polymer formation without the formation of low molecular weight products. A molecule containing a multiple bond is used as a monomer. In the polymerization of ethylene, the role of bifunctional structural unit plays a double bond, which, under the influence of an initiator (for example, organic benzyl peroxide (C 6 H 5 COO) 2), easily transforms into the radical state R; addition of a radical creates conditions for chain growth
The polymerization reaction is characterized by three stages: initiation, chain growth and chain termination:
open circuit
polymer electrical copper
This type of polymerization is called radical.
Polymerization can be initiated by cations or anions (ions). Ionic polymerization includes the same stages (initiation, chain propagation, chain termination). The initiators of cationic polymerization can be H+, inorganic aprotic acids SnCl 4, AlCl 3, and organometallic compounds Al(C 2 H 5) 3. The initiators of anionic polymerization are usually electron-donating compounds ( alkali metals, their alcoholates, etc.).
Cationic polymerization:
Polymerization can occur between different monomers. Such compounds are called copolymers. In table 1 shows examples of polymers and copolymers obtained by polymerization reactions.
Table 1 The most important polymers and copolymers
Copolymerization reactions
Let us consider the features of the radical copolymerization process. In the case of copolymerization of molecules A and B with the formation of radicals centered on molecules A or B of the growing chain, 4 stages of chain growth must take place:
So, in the case of radical polymerization, we are dealing with the distribution of products by molecular weight and a multi-route process with an infinitely large number of routes. Reaction products Pi are formed in the growth stages during chain transfer to the monomer.
The second way of formation of products (polymer molecules) is the stage of chain termination at X i and X j.
Polycondensation reactions
IN general view The scheme of the main polycondensation-chain growth reaction can be represented as follows:
(n and m are any integer, including one, X and Y are the original functional groups, A is a low molecular weight polycondensation product). In this case, the interaction of monomers with each other or with the resulting oligomers and the latter with each other obeys practically the same laws.
Since during polycondensation the monomers are exhausted even at low degrees of reaction completion, the growth of the high-molecular-weight polymer chain occurs predominantly. as a result of repeated connection of oligomeric or polymer molecules with each other at the terminal functional groups (the principle of multiple doubling), while the number of molecules in the system decreases (this is the stepwise nature of polycondensation). The number of initial functional groups - reaction (active) centers - also decreases during polycondensation, although in some cases the bonds formed during polycondensation react both with each other and with the initial reaction centers. The growth of the polymer chain during equilibrium polycondensation is accompanied by a reverse reaction of the polymer with the released low-molecular-weight product, which limits molecular weight polymer.
Polycondensation is accompanied by the formation of a polymer and a low molecular weight compound (H 2 O, HCl, NH 3, etc.). Monomers must contain at least two functional groups.
A typical polycondensation reaction underlies the production of phenol-formaldehyde resins
Almost all high molecular weight substances are polymers.
Polymers- these are substances whose molecules consist of a huge number of repeating structural units connected to each other by chemical bonds.
Polymers can be produced through reactions that can be divided into two main types: these are polymerization reactions And polycondensation reactions.
Polymerization reactions
Polymerization reactions - These are reactions of polymer formation by combining a huge number of molecules of a low molecular weight substance (monomer).
Number of monomer molecules ( n), combining into one polymer molecule, are called degree of polymerization.
Compounds with multiple bonds in molecules can enter into a polymerization reaction. If the monomer molecules are identical, then the process is called homopolymerization, and if different - copolymerization.
Examples of homopolymerization reactions, in particular, are the reaction of the formation of polyethylene from ethylene:
An example of a copolymerization reaction is the synthesis of styrene-butadiene rubber from 1,3-butadiene and styrene:
Polymers produced by the polymerization reaction and starting monomers
Monomer |
The resulting polymer |
||
Structural formula |
Name options |
Structural formula |
Name options |
| ethylene, ethene | polyethylene | ||
| propylene, propene | polypropylene | ||
| styrene, vinylbenzene | polystyrene, polyvinylbenzene | ||
| vinyl chloride, vinyl chloride, chlorethylene, chloroethene | polyvinyl chloride (PVC) | ||
| tetrafluoroethylene (perfluoroethylene) | teflon, polytetrafluoroethylene | ||
| isoprene (2-methylbutadiene-1,3) | isoprene rubber (natural) | ||
| butadiene-1,3 (divinyl) | butadiene rubber, polybutadiene-1,3 | ||
|
chloroprene(2-chlorobutadiene-1,3) |
chloroprene rubber | ||
|
butadiene-1,3 (divinyl) styrene (vinylbenzene) |
styrene butadiene rubber | ||
Polycondensation reactions
Polycondensation reactions- these are reactions of the formation of polymers from monomers, during which, in addition to the polymer, a low molecular weight substance (most often water) is also formed as a by-product.
Polycondensation reactions involve compounds whose molecules contain any functional groups. In this case, polycondensation reactions, according to whether one monomer or more is used, similar to polymerization reactions, are divided into reactions homopolycondensation And copolycondensation.
Homopolycondensation reactions include:
- * formation (in nature) of polysaccharide molecules (starch, cellulose) from glucose molecules:
- * reaction of formation of capron from ε-aminocaproic acid:
Copolycondensation reactions include:
- * reaction of formation of phenol-formaldehyde resin:
- * reaction of formation of lavsan (polyester fiber):
Polymer-based materials
Plastics
Plastics- materials based on polymers that are capable of being molded under the influence of heat and pressure and maintaining a given shape after cooling.
In addition to the high molecular weight substance, plastics also contain other substances, but the main component is still the polymer. Thanks to its properties, it binds all components into a single whole mass, and therefore it is called a binder.
Depending on their relationship to heat, plastics are divided into thermoplastic polymers (thermoplastics) And thermosets.
Thermoplastics- a type of plastic that can repeatedly melt when heated and solidify when cooled, making it possible to repeatedly change their original shape.
Thermosets- plastics, the molecules of which, when heated, are “stitched” into a single three-dimensional mesh structure, after which it is no longer possible to change their shape.
For example, thermoplastics are plastics based on polyethylene, polypropylene, polyvinyl chloride (PVC), etc.
Thermosets, in particular, are plastics based on phenol-formaldehyde resins.
Rubbers
Rubbers- highly elastic polymers, the carbon skeleton of which can be represented as follows:
As we see, rubber molecules contain double C=C bonds, i.e. Rubbers are unsaturated compounds.
Rubbers are obtained by polymerization of conjugated dienes, i.e. compounds in which two double C=C bonds are separated from each other by one single C-C bond.
1) butadiene:
In general terms (showing only the carbon skeleton), the polymerization of such compounds to form rubbers can be expressed by the following scheme:
Thus, based on the presented diagram, the isoprene polymerization equation will look like this:
| A very interesting fact is that it was not the most advanced countries in terms of progress that first became acquainted with rubber, but the Indian tribes, who lacked industry and scientific and technological progress as such. Naturally, the Indians did not obtain rubber artificially, but used what nature gave them: in the area where they lived ( South America), the Hevea tree grew, the sap of which contains up to 40-50% isoprene rubber. For this reason, isoprene rubber is also called natural, but it can also be obtained synthetically.
All other types of rubber (chloroprene, butadiene) are not found in nature, so they can all be characterized as synthetic. |
However, rubber, despite its advantages, also has a number of disadvantages. For example, due to the fact that rubber consists of long, chemically unrelated molecules, its properties make it suitable for use only in a narrow temperature range. In the heat, rubber becomes sticky, even slightly runny and smells unpleasant, and when low temperatures susceptible to hardening and cracking.
The technical characteristics of rubber can be significantly improved by vulcanization. Vulcanization of rubber is the process of heating it with sulfur, as a result of which individual, initially unconnected, rubber molecules are “stitched” together with chains of sulfur atoms (polysulfide “bridges”). The scheme for converting rubbers into rubber using synthetic butadiene rubber as an example can be demonstrated as follows:
Fibers
Fibers are materials based on polymers of a linear structure, suitable for the manufacture of threads, tows, and textile materials.
Classification of fibers according to their origin
Man-made fibers(viscose, acetate fiber) are obtained by chemical treatment of existing natural fibers (cotton and flax).
Synthetic fibers are obtained mainly by polycondensation reactions (lavsan, nylon, nylon).