Lesson “Hydrogen sulfide. Sulfides"
(9th grade)
Lesson objectives:
Educational:
– Consider the composition, structure and properties of hydrogen sulfide.
- Learn to write reaction equations characterizing the properties of hydrogen sulfide and qualitative reactions to sulfides.
– Consider the impact of hydrogen sulfide on the environment and human health.
Educational:
Be able to apply acquired knowledge to explain a variety of chemical phenomena and properties of substances.
Be able to use additional material from information sources, computer technologies
Use acquired knowledge and skills in practical activities and everyday life: a) environmentally literate behavior in the environment; b) assessing the impact of chemical environmental pollution on the body person.
Educational:
– Students' caring attitude towards the environment and their health.
- Developing the ability to work in pairs during self-analysis of control sections and tests.
Lesson objectives:
Promote the development of chemical literacy in students.
Interdisciplinary connections: The connection of chemistry with other sciences: biology, geography, mathematics, medicine and literature.
Lesson type: learning a new topic.
Elements of pedagogical technologies: differentiated learning, problem-based learning, ICT, gaming technologies.
Methods:
reproductive, partially search.
verbal (story, conversation), independent work of students.
Equipment and facilities:
multimedia screen
Personal Computer
reagents for carrying out qualitative reactions on sulfide anion
textbook
During the classes
I Organizational moment (2 min.)
Receiving a report from the duty officer;
Greetings
Hello guys! Today we have guests at our lesson. Don't worry, work as usual.
II Repetition of previously studied material. Checking homework
(10 min.)
?
Let's remember what we studied in the last lesson.
We learned that sulfur is a simple substance, we studied its physical and chemical properties, allotropic modifications, and the occurrence of sulfur in nature.
At home, it was necessary to consider the proposed reactions in the light of ideas about redox processes.
Did everyone complete the written task?
Conducting differentiated written work (5-7 min.)
Assistants distribute tasks according to options.
Students answer differentiated written work questions.
Mutual verification of work completion with simultaneous presentation of answers on the slide.
Who worked with level B and C - raise your hands.
Slide No. 1
III Learning new material (30 min.)
Mystery
I am everywhere - but little by little,
I blacken the silver spoon.
When an egg is spoiled
I'm also immediately obvious
I'm curbing my appetite
And very poisonous.
And remember the lines from A.S. Pushkin, written in 1832 in the poem “And then we went - and fear embraced me”:
“...Then I heard (oh, marvel!) a foul smell,
It’s like a rotten egg has broken..."
?
What connection does Pushkin mention in this passage of this verse?How did you guess that it was hydrogen sulfide?
What properties of hydrogen sulfide are still unknown to you?
So, the topic of the lesson today is hydrogen sulfide(open the board) .
We write down the topic “Hydrogen sulfide. Sulfides ».
Slide No. 2
Lesson objectives: Slide No. 3
Study the composition, structure and properties, methods of producing hydrogen sulfide and sulfides;
Trace the cause-and-effect relationship between the structure, properties and use of substances;
Consider the impact of hydrogen sulfide on the environment and human health;
Strengthen the ability to compile chemical reaction processes and consider them from the point of view of redox processes;
Promote student literacy development.
Plan for discussing this topic on the board.
As we study the topic, we will take notes.
1. Being in nature
Slide No. 4
Hydrogen sulfide is quite common in nature. And where exactly, he will tell us(student's speech)
Hydrogen sulfide occurs wherever decomposition and rotting of plant and, especially, animal remains occurs under the influence of microorganisms.
Some photosynthetic bacteria, such as green sulfur bacteria, for which hydrogen sulfide is a nutrient, produce elemental sulfur, a product of the oxidation of hydrogen sulfide.
In our country, hydrogen sulfide is found in the Caucasus in sulfur mineral springs. Near Mineralnye Vody there is the only hydrogen sulfide spring in Russia and in the world, unique in its chemical composition, which has restored health to many people. (The famous resorts are Pyatigorsk, Essentuki, Matsestinsky springs.
The sources are used to treat diseases of the musculoskeletal system, cardiovascular system, and skin diseases. Hydrogen sulfide irritates the nerve endings of the skin, dilating small blood vessels, improving blood circulation in the tissues, i.e. produces their food. It also normalizes blood pressure, the nervous system, and improves heart function.
Hydrogen sulfide is found in volcanic gases.
It is maintained in a dissolved state in the waters of the Black Sea.
2. Production of hydrogen sulfide (see textbook)
Slide No. 5
Hydrogen sulfide is obtained:
In laboratory conditions, during the interaction of iron sulfide (II) with hydrochloric acidH 2 SO 4
FeS+H 2 SO 4 = Fe SO 4 +H 2 S
Passing hydrogen over molten sulfur
H 2 + S = H 2 S
Interaction of aluminum sulfide with water (the purest hydrogen sulfide)
Al 2 S 3 + 6H 2 O = 2Al(OH) 3 ↓ + 3H 2 S
When heating a mixture of paraffin and sulfur
C 20 H 42 + 21 S = 21 H 2 S + 20 C
Once at a lecture they demonstrated an experiment: melting sulfur in a test tube. Suddenly everyone smelled a disgusting smell. The lecture was disrupted. Everything turned out to be simple: pieces of paraffin from the cork lid of the bottle in which the sulfur powder was stored fell into the test tube with sulfur. When this mixture was heated, hydrogen sulfide was released.
If heating is stopped, the reaction stops and hydrogen sulfide is not released. This fact is convenient to use in educational laboratories.
And now we will have a little physical education.
3 Structure of hydrogen sulfide
Slide No. 6
Let's look at the structure of hydrogen sulfide (type of chemical bond, type of crystal lattice).
?
You know that the properties of substances depend on the composition and structure.What physical properties do you assume based on the structure (MCR)?
This:Slide No. 7
Gas;
With low melting point (-82 0 C) and boiling point (-60 0 WITH);
Colorless;
With the smell of rotten eggs and a sweetish taste;
Slightly soluble in water (highly soluble in alcohol);
(2.4 volumes of hydrogen sulfide are dissolved in 1 volume of water)
(This solution is called hydrogen sulfide water or hydrosulfide acid)
Heavier than air;
POISONOUS!
Even one breath of pure hydrogen sulfide leads to loss of consciousness due to paralysis of the respiratory center. Hydrogen sulfide is able to interact with iron ions included in the hemoglobin of the blood.
?
Slide No. 8A problem arises : is hydrogen sulfide beneficial or harmful?
Hydrogen sulfide is poisonous, but there are hydrogen sulfide healing sources.
We must solve this problem by the end of the lesson.
4 Chemical properties of hydrogen sulfide
Slide No. 9
a) burns with a bluish flame (at a temperature of 250 0 – 300 0 WITH)
2 H 2 S -2 + 3 O 2 0 = 2 S +4 O 2 + 2 H 2 O
(brief analysis of OVR)
b) with a lack of oxygen
2 H 2 S + O 2 = 2 S 0 ↓+ 2 H 2 O
(reducing agent)
What properties does hydrogen sulfide exhibit in these reactions?
Analysis
When dissolved in water, hydrosulfide acid is formed.
?
Describe this acidSlide No. 10Weak;
Dibasic;
Oxygen-free.
Dissociation occurs in two stages:
IH 2 S → H + + H.S. - (hydrosulfide ion is formed)
IIH.S. - ↔ H + + S 2- (in the second step, dissociation practically does not occur)
?
What salts does hydrosulfide acid form?
medium (sulfides) –Na 2 S
acidic (hydrosulfides) –NaHS
?
Hydrogen sulfide acid has the general properties of acids. Which ones?Interaction with bases, basic oxides, salts
Let us write down the chemical equation for the interaction of hydrogen sulfide acid with sodium hydroxide.
H 2 S+2NaOH ( hut ) → Na 2 S+2H 2 O
H 2 S ( hut ) + 2NaOH → NaHS + 2H 2 OSlide №11
Write down chemical reactions with basic oxides and salts at home.
?
Suggest a reaction to detect sulfide anionS 2-Conduct a laboratory experiment to confirm.Slide No. 12
Write the UCR in molecular and ionic form.
Many sulfides are insoluble in water and are colored:
- PbS- black color;Slide No. 13
- CuS- black color;
- AgS– black color (silver items turn black when stored for a long time in the presence of hydrogen sulfide in the air);
- ZnS- White color;
- MgS- pink color.
Hydrogen sulfide and hydrosulfide acid are used in analytical chemistry to precipitate heavy metals.
?
Let's get back to our problem.Is hydrogen sulfide beneficial or harmful?
5 Use of hydrogen sulfide
Slide number 14
Hydrogen sulfide is of limited use due to its toxicity.
In analytical chemistry, hydrogen sulfide and hydrogen sulfide water are used as reagents for the precipitation of heavy metals, the sulfides of which are very slightly soluble.
In medicine - as part of natural and artificial hydrogen sulfide baths, as well as in some mineral waters.
Hydrogen sulfide is used to produce sulfuric acid, elemental sulfur, and sulfides.
Colored sulfides serve as the basis for the manufacture of paints. They are also used in analytical chemistry.
Potassium, strontium and barium sulfides are used in tanning to remove wool from hides before tanning.
In recent years, the possibility of using hydrogen sulfide accumulated in the depths of the Black Sea as energy (hydrogen sulfide energy) and chemical raw materials has been considered.
?
Is everything now clear about the mystery of hydrogen sulfide?Student statements
Why doesn't hydrogen sulfide accumulate in large quantities in nature?
(it is oxidized by atmospheric oxygen to elemental sulfur)
6 Final part (3 min.)
Slide number 15
What new things did we learn during the lesson?
What can be practically applied in life?
Student answers
Homework: §11, ex. 2, 3 page 34
Creative task (optional) : WhyDo artistic paintings by old masters darken over time and lose their original brightness? How do restorers update these paintings?
Atmospheric pollution causes blackening of the surface of paintings painted with oil paints that contain lead white. One of the main reasons for the darkening of artistic paintings by old masters was the use of lead white, which over several centuries, interacting with traces of hydrogen sulfide in the air (formed in small quantities during the rotting of proteins; in the atmosphere of industrial regions, etc.) turns intoPbS. Lead white is a pigment that is lead carbonate (II). It reacts with hydrogen sulfide contained in the polluted atmosphere, forming lead sulfide (II), black connection:
PbCO 3 + H 2 S = PbS↓ + CO 2 + H 2 O
When processing lead sulfide (II) with hydrogen peroxide the reaction occurs:
PbS + 4 H 2 O 2 = PbSO 4 + 4 H 2 O,
this produces lead sulfate (II), the connection is white.
This is how blackened oil paintings are restored.
Slide 1
Slide 2
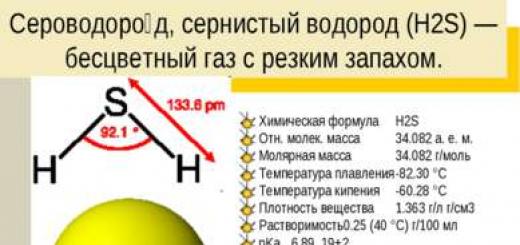
 Hydrogen sulfide, hydrogen sulfide (H2S) is a colorless gas with a pungent odor. Chemical formula H2S Rel. molecular mass 34.082 a. e.m. Molar mass 34.082 g/mol Melting point -82.30 °C Boiling point -60.28 °C Density of the substance 1.363 g/l g/cm3 Solubility 0.25 (40 °C) g/100 ml pKa 6.89, 19±2 State ( st.conv) colorless gas CAS number 7782-79-8
Hydrogen sulfide, hydrogen sulfide (H2S) is a colorless gas with a pungent odor. Chemical formula H2S Rel. molecular mass 34.082 a. e.m. Molar mass 34.082 g/mol Melting point -82.30 °C Boiling point -60.28 °C Density of the substance 1.363 g/l g/cm3 Solubility 0.25 (40 °C) g/100 ml pKa 6.89, 19±2 State ( st.conv) colorless gas CAS number 7782-79-8
Slide 3
 Occurrence in nature Occurs naturally in petroleum, natural gas, volcanic gas and in hot springs.
Occurrence in nature Occurs naturally in petroleum, natural gas, volcanic gas and in hot springs.
Slide 4
 Properties Thermally unstable (at temperatures above 400 °C it decomposes into simple substances - S and H2), a poisonous gas heavier than air with an unpleasant odor of rotten eggs. The hydrogen sulfide molecule has an angular shape, so it is polar (μ = 0.34 10-29 C m). Unlike water molecules, hydrogen sulfide molecules do not form strong hydrogen bonds, which is why H2S is a gas. A saturated aqueous solution of H2S is hydrosulfide acid.
Properties Thermally unstable (at temperatures above 400 °C it decomposes into simple substances - S and H2), a poisonous gas heavier than air with an unpleasant odor of rotten eggs. The hydrogen sulfide molecule has an angular shape, so it is polar (μ = 0.34 10-29 C m). Unlike water molecules, hydrogen sulfide molecules do not form strong hydrogen bonds, which is why H2S is a gas. A saturated aqueous solution of H2S is hydrosulfide acid.
Slide 5
 Preparation In the laboratory it is usually obtained by the action of dilute acids on sulfides: FeS + 2HCl = FeCl2 + H2S Or by adding water to aluminum sulfide: Al2S3 + H2O = 2Al(OH)3 + H2S (the reaction differs in the purity of the resulting hydrogen sulfide)
Preparation In the laboratory it is usually obtained by the action of dilute acids on sulfides: FeS + 2HCl = FeCl2 + H2S Or by adding water to aluminum sulfide: Al2S3 + H2O = 2Al(OH)3 + H2S (the reaction differs in the purity of the resulting hydrogen sulfide)
Slide 6
 Application Hydrogen sulfide is of limited use due to its toxicity. In analytical chemistry, hydrogen sulfide and hydrogen sulfide water are used as a reagent for the precipitation of heavy metals, the sulfides of which are very slightly soluble. In medicine - as part of hydrogen sulfide baths. Hydrogen sulfide is used to obtain sulfuric acid, elemental sulfur, sulfides. Used in organic synthesis to obtain thiophene and mercaptans. In recent years The possibility of using hydrogen sulfide accumulated in the depths of the Black Sea as an energy and chemical raw material is being considered.
Application Hydrogen sulfide is of limited use due to its toxicity. In analytical chemistry, hydrogen sulfide and hydrogen sulfide water are used as a reagent for the precipitation of heavy metals, the sulfides of which are very slightly soluble. In medicine - as part of hydrogen sulfide baths. Hydrogen sulfide is used to obtain sulfuric acid, elemental sulfur, sulfides. Used in organic synthesis to obtain thiophene and mercaptans. In recent years The possibility of using hydrogen sulfide accumulated in the depths of the Black Sea as an energy and chemical raw material is being considered.
Slide 7
 Toxicology Very toxic. At high concentrations, a single inhalation can cause instant death. At small concentrations, adaptation to the unpleasant smell of “rotten eggs” occurs quite quickly, and it ceases to be felt. A sweetish metallic taste appears in the mouth. At high concentrations it has no odor.
Toxicology Very toxic. At high concentrations, a single inhalation can cause instant death. At small concentrations, adaptation to the unpleasant smell of “rotten eggs” occurs quite quickly, and it ceases to be felt. A sweetish metallic taste appears in the mouth. At high concentrations it has no odor.
Class: 9
Presentation for the lesson


















 Back forward
Back forward
Attention! Slide previews are for informational purposes only and may not represent all the features of the presentation. If you are interested in this work, please download the full version.
“Then I heard (oh, marvel!), a bad smell,
It's like a rotten egg has broken,
Or the quarantine guard was smoking with a sulfur brazier.
I held my nose and turned my face away..."
Pushkin A.S.
Lesson objectives:
Educational:
– To consolidate students’ knowledge on the topic covered: allotropy of sulfur, physical and chemical properties, use of sulfur, occurrence in nature.
– Consider the properties of the hydrogen sulfide compound and its salts. – Consider the impact of hydrogen sulfide on the environment and human health.
Educational:
– be able to compose reaction equations in molecular form and from the point of view of redox processes
Educational:
– Moral and aesthetic education of students towards the environment.
Equipment:
- Textbook “Chemistry 9th grade” G.E. Rudzitis, F.G. Feldman.
- Kipa apparatus for producing hydrogen sulfide.
- Multimedia projector.
- On the students' desks are reagents for recognizing sulfide ions.
- (Laboratory experiment No. 5 p. 43).
- Presentation for the lesson.
During the classes
1. Organizational moment. Checking homework.
Exercise 5 (4 students prepare at the board).
Frontal conversation:
– Describe the physical properties of sulfur.
– Explain the essence of allotropy. What are the causes of allotropy in sulfur? What are the causes of allotropy in oxygen?
– Where is sulfur found in nature? List what natural sulfur compounds you know?
Question for students at the board:
– What oxidation states does sulfur have in each of these compounds?
– How does sulfur interact with metals?
– How does sulfur interact with non-metals?
– In what cases does sulfur act as an oxidizing agent, and in some cases as a reducing agent?
Assignment for the whole class: Write the interaction of sulfur and hydrogen, indicate the oxidizing agent and the reducing agent.
2. Studying new material.
Filling out the table:
| Properties | Hydrogen sulfide |
| Chemical formula of the substance | |
| Type of chemical bond | |
| Physical state at no. | |
| Color | |
| Density by air | |
| Smell | |
| Solubility in water (dissociation equation) | |
| Physiological action | |
| Being in nature | |
| Preparation in the laboratory (reaction equation) | |
| Redox properties | |
| Acid-base properties | |
| Qualitative reaction to sulfide ions | |
| Application | |
| Environmental pollution |
– What is the name of the resulting substance?
– What do you know about this substance?
– What kind of connection is formed?
Slide. Molecular formula. Type of chemical bond
Slide. Being in nature.
– Is hydrogen sulfide lighter or heavier than air? (Calculation of molecular weight).
Slide. Air density determination
Demonstration of hydrogen sulfide production.
The teacher and the students discuss the physical properties of hydrogen sulfide:
Assignment: write the reaction equation for producing hydrogen sulfide.
Slide. Reaction to produce hydrogen sulfide
Slide. Physical properties.
Teacher: H 2 S is a strong reducing agent. For example, when standing in air for a long time, hydrogen sulfide water becomes cloudy, this is explained by the interaction of H 2 S with oxygen in the air, and elemental sulfur is released.
(Demonstration of pre-prepared hydrogen sulfide water.)
H 2 S burns in air with a blue flame, producing sulfur dioxide, or sulfur (IV) oxide.
Consolidation.
Performing exercise 1 on page 34
Slide. An aqueous solution of hydrogen sulfide exhibits the properties of a weak acid.
Write an equation for its dissociation.
Slide. Dissociation equation.
Teacher: Hydrogen sulfide acid exhibits all the general properties of acids.
Question: What properties of acids do we know?
Slide. Properties of acids
At home, write down equations for all the listed reactions. In molecular and ionic form
Hydrogen sulfide acid reacts with alkalis in a neutralization reaction, forming 2 series of salts: hydrosulfides and sulfides.
Students perform a laboratory experiment and write down the reaction equation. (if they don’t have time, then they complete the ionic reaction at home. (Working with the solubility table)..
Conclusion: Only sulfides of alkali metals and ammonium are highly soluble in water. Sulfides of other metals are practically insoluble in water; they precipitate when a solution of ammonium sulfide (NH 4) 2 S is introduced into solutions of metal salts. Many sulfides are colored: CdS - bright yellow; CuS PbS – black; SnS – orange; HgS – red.
Therefore, reactions to form insoluble sulfides can be used to detect specific ions (i.e. they are qualitative).
Slide. The impact of hydrogen sulfide on the environment and human health.
Slide. Application.
House task:
§11 page 34 No. 2 and draw up reaction equations in molecular and ionic form, which were discussed in class. (Finish filling out the table).
Purpose of the lesson: To consolidate students’ knowledge on the topic covered: allotropy of sulfur, physical and chemical properties, use of sulfur, occurrence in nature. Consider the properties of the sulfur compound - hydrogen sulfide and its salts. Consider the impact of hydrogen sulfide on the environment and human health. Consider the impact of hydrogen sulfide on the environment and human health. be able to draw up reaction equations in molecular form and from the point of view of redox processes. Moral and aesthetic education of students towards the environment.
“Then I heard (oh, marvel!), a foul smell, As if a rotten egg had broken, Or a quarantine guard was smoking chamois with a brazier. I, holding my nose, turned my face away...” Pushkin A.S.

Properties Hydrogen sulfide Chemical formula of the substance Type of chemical bond Physical state at no. Color Density in air Odor Occurrence in nature Solubility in water (dissociation equation) Obtained in the laboratory (reaction equation) Redox properties Acid-base properties Qualitative reaction to sulfide ions Physiological effect Environmental pollution Application

Molecular formula H 2 S oxidation state of sulfur (-2). Covalent polar bond The hydrogen sulfide molecule has an angular shape, so it is polar. Unlike water molecules, the hydrogen atoms in the molecule do not form strong hydrogen bonds, which is why hydrogen sulfide is a gas.


In a free state, it is found in the composition of volcanic gases, in many springs of volcanic areas, it is part of volcanic ash in a dissolved and partly in a free state, hydrogen sulfide is found in the Black Sea, starting from a depth of 200 meters or more. Hydrogen sulfide is found in a dissolved and partly free state in the Black Sea, starting from a depth of 200 meters or more. it is formed in small quantities wherever decomposition or decay of organic matter occurs: it is present in mineral muds formed at the bottom of shallow salt lakes; in the form of mixed substances of oil and gas. For some microorganisms (sulfur bacteria), hydrogen sulfide is not a poison, but a nutrient. Assimilating hydrogen sulfide, they release free sulfur. Such deposits form at the bottom of lakes on the northern coast of Africa, in Cyrenaica near the city of Benghazi.

Where does hydrogen sulfide come from in the Black Sea? Hydrogen sulfide is constantly formed at the bottom of the Black Sea during the interaction of sulfates dissolved in sea water with organic substances: Hydrogen sulfide is constantly formed at the bottom of the Black Sea during the interaction of sulfates dissolved in sea water with organic substances: CaSO 4 + CH 4 => CaS + CO 2 + 2H 2 O CaSO 4 + CH 4 => CaS + CO 2 + 2H 2 O CaS + H 2 O + CO 2 => CaCO 3 + H 2 S Sulfate-reducing bacteria participate in these reactions. Hydrogen sulfide does not reach the upper layers of water, since at a depth of about 150 m it encounters oxygen penetrating from above. At the same depth, sulfur bacteria live, which help oxidize hydrogen sulfide to sulfur: 2H 2 S + O 2 => 2H 2 O + 2S 2H 2 S + O 2 => 2H 2 O + 2S In recent years, due to the catastrophic pollution of the Black Sea, the upper The limit of hydrogen sulfide gradually rises, killing all living things on its way. The deadly border has already reached a depth of 40 m. CaS + CO 2 + 2H 2 O CaSO 4 + CH 4 => CaS + CO 2 + 2H 2 O CaS + H 2 O + CO 2 => CaCO 3 + H 2 S Sulfate-reducing bacteria participate in these reactions. Hydrogen sulfide does not reach the upper layers of water, since at a depth of about 150 m it encounters oxygen penetrating from above. At the same depth, sulfur bacteria live, which help oxidize hydrogen sulfide to sulfur: 2H 2 S + O 2 => 2H 2 O + 2S 2H 2 S + O 2 => 2H 2 O + 2S In recent years, due to the catastrophic pollution of the Black Sea, the upper The limit of hydrogen sulfide gradually rises, killing all living things on its way. The deadly border has already reached a depth of 40 m."> 

FeSO 4 + H 2 S FeS + H 2 SO 4 => FeSO 4 + H 2 S 2. By synthesis from sulfur and hydrogen: H 2 + S =>" title="Hydrogen sulfide can be obtained 1. In the laboratory, hydrogen sulfide obtained by reacting iron sulfide with hydrochloric or dilute sulfuric acid: FeS + H 2 SO 4 => FeSO 4 + H 2 S FeS + H 2 SO 4 => FeSO 4 + H 2 S 2. Synthesis from sulfur and hydrogen: H 2 + S =>" class="link_thumb"> 10 !} Hydrogen sulfide can be obtained 1. In the laboratory, hydrogen sulfide is obtained by reacting iron sulfide with hydrochloric or dilute sulfuric acids: FeS + H 2 SO 4 => FeSO 4 + H 2 S FeS + H 2 SO 4 => FeSO 4 + H 2 S 2. Synthesis from sulfur and hydrogen: H 2 + S => H 2 S H 2 + S => H 2 S 3. The interaction of aluminum sulfide with water aluminum with water (this reaction differs (this reaction differs in the purity of the resulting hydrogen sulfide): the purity of the resulting hydrogen sulfide): Al 2 S 3 +6H 2 O => 3H 2 S+2Al(OH) 3 FeSO 4 + H 2 S FeS + H 2 SO 4 => FeSO 4 + H 2 S 2. Synthesis from sulfur and hydrogen: H 2 + S =>"> FeSO 4 + H 2 S FeS + H 2 SO 4 => FeSO 4 + H 2 S 2. By synthesis from sulfur and hydrogen: H 2 + S => H 2 S H 2 + S => H 2 S 3. By the interaction of aluminum sulfide with water aluminum with water (this reaction is different (this reaction is different in purity obtained hydrogen sulfide): purity of the obtained hydrogen sulfide): Al 2 S 3 +6H 2 O => 3H 2 S+2Al(OH) 3"> FeSO 4 + H 2 S FeS + H 2 SO 4 => FeSO 4 + H 2 S 2. By synthesis from sulfur and hydrogen: H 2 + S =>" title="Hydrogen sulfide can be obtained 1. In the laboratory, hydrogen sulfide is obtained by reacting iron sulfide with hydrochloric or dilute sulfuric acids: FeS + H 2 SO 4 => FeSO 4 + H 2 S FeS + H 2 SO 4 => FeSO 4 + H 2 S 2. Synthesis from sulfur and hydrogen: H 2 + S =>"> title="Hydrogen sulfide can be obtained 1. In the laboratory, hydrogen sulfide is obtained by reacting iron sulfide with hydrochloric or dilute sulfuric acids: FeS + H 2 SO 4 => FeSO 4 + H 2 S FeS + H 2 SO 4 => FeSO 4 + H 2 S 2. Synthesis from sulfur and hydrogen: H 2 + S =>"> !}
Convenient way. Once at a lecture an experiment was demonstrated: melting sulfur in a test tube. Suddenly everyone smelled a disgusting smell. The lecture was disrupted. Once at a lecture an experiment was demonstrated: melting sulfur in a test tube. Suddenly everyone smelled a disgusting smell. The lecture was disrupted. Everything turned out to be simple: pieces of paraffin from the cork lid of the bottle in which the sulfur powder was stored fell into the test tube with sulfur. When heated, a mixture of paraffin and sulfur releases hydrogen sulfide: Everything turned out to be simple: pieces of paraffin from the cork lid of the bottle in which the sulfur powder was stored fell into the test tube with sulfur. When heated, a mixture of paraffin and sulfur releases hydrogen sulfide: C 20 H S => 21H 2 S + 20C C 20 H S => 21H 2 S + 20C The more the mixture is heated, the more the mixture is heated, the more active the gas is released. If heating is stopped, If heating is stopped, the reaction stops and hydrogen sulfide is not released. and hydrogen sulfide is not released. Therefore, the reaction is very convenient for producing hydrogen sulfide in educational laboratories. in teaching laboratories. 21H 2 S + 20C C 20 H 42 + 21S => 21H 2 S + 20C The more the mixture is heated, the more the mixture is heated, the more gas is released. If heating is stopped, If heating is stopped, the reaction stops and hydrogen sulfide is not released. and hydrogen sulfide is not released. Therefore, the reaction is very convenient for producing hydrogen sulfide in educational laboratories. in educational laboratories."> 
Physical properties of sulfur Hydrogen sulfide (hydrogen sulfide, hydrogen sulfide) is a colorless gas with the smell of rotten eggs and a sweetish taste. Poorly soluble in water, good in ethanol. Poisonous. Thermally unstable (at temperatures above 400 °C it decomposes into simple substances S and H2). Hydrogen sulfide is slightly soluble in water. At t = 20 º, 2.4 volumes of hydrogen sulfide are dissolved in one volume of water; this solution is called hydrogen sulfide water or weak hydrogen sulfide acid. Hydrogen sulfide (hydrogen sulphide, hydrogen sulfide) is a colorless gas with the smell of rotten eggs and a sweetish taste. Poorly soluble in water, good in ethanol. Poisonous. Thermally unstable (at temperatures above 400 °C it decomposes into simple substances S and H2). Hydrogen sulfide is slightly soluble in water. At t = 20 º, 2.4 volumes of hydrogen sulfide are dissolved in one volume of water; this solution is called hydrogen sulfide water or weak hydrogen sulfide acid. A solution of hydrogen sulfide in water is a very weak hydrogen sulfide acid. A solution of hydrogen sulfide in water is a very weak hydrogen sulfide acid.




Qualitative reaction to sulfide ion Laboratory experiment Laboratory experiment Pb(NO 3) 2 + Na 2 S PbS + 2NaNO 3 black precipitate black precipitate (Na 2 S + CuCl 2 CuS + 2HCl) black precipitate black precipitate write the complete ionic and short ionic equation

Hydrogen sulfide has the properties of a reducing agent. Hydrogen sulfide burns in air with a blue flame and produces sulfur dioxide or sulfur oxide (IV) 2H 2 S O 2 2H 2 O + 2S +4 O 2 2H 2 S O 2 2H 2 O + 2S +4 O 2 S -2 -6е S +4 Reducing agent O 2 +4е 2O -2 Oxidizing agent When there is a lack of oxygen, water and sulfur vapors are formed: When there is a lack of oxygen, water and sulfur vapors are formed: 2H 2 S -2 + O 2 2H 2 O + 2S 0 S -2 - 2e S 0 Reducing agent O 2 +4e 2O -2 Oxidizing agent O 2 +4e 2O -2 Oxidizing agent Hydrogen sulfide has the properties of a reducing agent: if a small amount of iodine water is added to a test tube with hydrogen sulfide, the solution will become discolored and sulfur H 2 S -2 will appear on the surface of the solution + I 0 2 S 0 + 2HI -1 S -2 -2e S 0 Reducing agent I e 2I -1 oxidizing agent I e 2I -1 oxidizing agent

Effect of hydrogen sulfide on the environment and human health Very toxic. Inhalation of air containing hydrogen sulfide causes dizziness, headache, nausea, and with significant concentrations leads to coma, convulsions, pulmonary edema and even death. At high concentrations, a single inhalation can cause instant death. At small concentrations, adaptation to the unpleasant smell of “rotten eggs” occurs quite quickly, and it ceases to be felt. A sweetish metallic taste appears in the mouth. At high concentrations, due to paralysis of the olfactory nerve, the smell of hydrogen sulfide is not felt. At high concentrations, due to paralysis of the olfactory nerve, the smell of hydrogen sulfide is not felt.

Application. Hydrogen sulfide is of limited use due to its toxicity. In analytical chemistry, hydrogen sulfide and hydrogen sulfide water are used as reagents for the precipitation of heavy metals, the sulfides of which are very slightly soluble. In medicine, as part of natural and artificial hydrogen sulfide baths, as well as in some mineral waters. Hydrogen sulfide is used to produce sulfuric acid, elemental sulfur, and sulfides. Used in organic synthesis to obtain thiophene and mercaptans. Colored sulfides serve as the basis for the manufacture of paints, including luminous ones. They are also used in analytical chemistry. Potassium, strontium and barium sulfides are used in tanning to remove wool from hides before tanning. Potassium, strontium and barium sulfides are used in tanning to remove wool from hides before tanning. In recent years, the possibility of using hydrogen sulfide accumulated in the depths of the Black Sea as energy (hydrogen sulfide energy) and chemical raw materials has been considered.