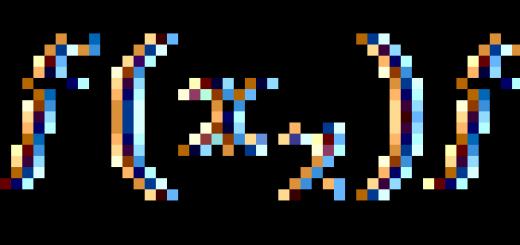What is meteoric iron? How does it appear on Earth? You will find answers to these and other questions in the article. Meteoritic iron is a metal found in meteorites and consisting of several mineral phases: taenite and kamacite. It makes up the majority of metallic meteorites, but is also found in other types. Consider meteoric iron below.
Structure
When a polished cut is etched, the structure of meteorite iron appears in the form of the so-called Widmanstätten figures: intersecting beams-strips (kamacite) bordered by shiny narrow ribbons (taenite). Sometimes you can see polygonal fields-platforms.
A fine-grained mixture of taenite and kamacite forms plessite. The iron we are considering in meteorites of the hexahedrite type, which is almost completely composed of kamacite, forms a structure in the form of parallel thin lines, called non-man.
Application
In ancient times, people did not know how to make metal from ore, so meteorite iron was its only source. It has been proven that elementary tools from this substance (identical in shape to stone ones) were created as early as the Bronze Age and the Neolithic. A dagger found in the tomb of Tutankhamen and a knife from the Sumerian town of Ur (about 3100 BC) were made from it, beads found 70 km from Cairo, in places of eternal rest, in 1911 (about 3000 BC). n. e.).

Tibetan sculpture has also been created from this substance. It is known that the king (Ancient Rome) had a metal shield made from "a stone that fell from the sky." In 1621, for Jahangir (the ruler of an Indian principality), a dagger, two sabers and a spearhead were forged from heavenly iron.
A saber made of this metal was presented to Tsar Alexander I. According to legend, Tamerlane's swords also had a cosmic origin. Today, heavenly iron is used in jewelry production, but most of it is used for scientific experiments.
meteorites
Meteorites are 90% metal. Therefore, the first person began to use heavenly iron. How to distinguish it from the earth? This is very easy to do, because it contains about 7-8% nickel impurities. It is not for nothing that in Egypt it was called stellar metal, and in Greece - heavenly. This substance was considered very rare and expensive. It's hard to believe, but it was previously framed in gold frames.

Stellar iron is not resistant to corrosion, so products made from it are rare: they simply could not survive to this day, as they crumbled from rust.
According to the method of detection, iron meteorites are divided into falls and finds. Falls are called such meteorites, the decline of which was visible and which people were able to find shortly after they landed.
Finds are meteorites found on the surface of the Earth, but no one observed their fall.
meteorites falling
How does a meteorite fall to Earth? Today, more than a thousand falls of heavenly wanderers have been recorded. This list includes only meteors whose passage through the earth's atmosphere was recorded by automatic equipment or observers.

Star rocks enter our planet's atmosphere at a speed of about 11-25 km/s. At this speed, they begin to warm up and glow. Due to ablation (charring and blowing off by a counter flow of particles of the meteorite substance), the weight of a body that has reached the Earth's surface can be less, and sometimes significantly less than its mass at the entrance to the atmosphere.
The fall of a meteorite to Earth is an amazing phenomenon. If the meteorite body is small, then at a speed of 25 km / s it will burn without residue. As a rule, out of tens and hundreds of tons of primary mass, only a couple of kilograms and even grams of substance reach the earth. Traces of combustion of celestial bodies in the atmosphere can be found throughout almost the entire trajectory of their fall.
The fall of the Tunguska meteorite

This mysterious event happened in 1908, on June 30th. How did the fall of the Tunguska meteorite occur? The celestial body fell in the Podkamennaya area at 7:15 a.m. local time. It was early in the morning, but we were already awake a long time ago. They were engaged in current affairs, which in the village courtyards require unceasing attention from the very sunrise.
The Podkamennaya Tunguska itself is a full-flowing and mighty river. It flows on the lands of the present Krasnoyarsk Territory, and originates in the Irkutsk region. It makes its way through the taiga wilderness areas, replete with wooded high banks. This is a godforsaken land, but it is rich in minerals, fish and, of course, impressive hordes of mosquitoes.
The mysterious event began at 6:30 local time. Residents of villages located along the banks of the Yenisei saw a fireball of impressive size in the sky. It moved from south to north, and then disappeared over the taiga. At 07:15 a bright flash lit up the sky. After a while there was a terrible roar. The earth shook, glass flew out of the windows in the houses, the clouds turned red. They kept that color for a couple of days.
Observatories located in different parts of the planet recorded a blast wave of great strength. Next, people wanted to know what happened and where. It is clear that in the taiga, but it is very large.
It was not possible to organize a scientific expedition, since there were no rich patrons who were ready to pay for such research. Therefore, scientists first decided only to interview eyewitnesses. They talked with Evenks and Russian hunters. They said that at first a strong wind blew and a loud whistle was heard. Further, the sky was filled with red light. After a thunderclap was heard, trees began to light up and fall. It got very hot. After a couple of seconds, the sky shone even more strongly, and the thunder rang out again. A second sun appeared in the sky, which was much brighter than the usual luminary.
These indications were all limited. Scientists decided that a meteorite fell in the Siberian taiga. And since he landed in the zone of Podkamennaya Tunguska, they called him Tunguska.
The first expedition was equipped only in 1921. Its initiators were academicians Fersman Alexander Evgenievich (1883-1945) and Vernadsky Vladimir Ivanovich (1863-1945). This journey was led by Kulik Leonid Alekseevich (1883-1942), the leading specialist of the USSR on meteorites. Then several more scientific campaigns were organized in 1927-1939. As a result of these studies, the assumptions of scientists were confirmed. In the basin of the Tunguska Podkamennaya River, a meteorite fell indeed. But the huge crater that the fallen body was supposed to create was not discovered. They did not find any crater at all, even the smallest one. But they found the epicenter of a powerful explosion.
It was installed on trees. They stood there as if nothing had happened. And around them, in a radius of 200 km, there was a fallen forest. The surveyors decided that the explosion happened at an altitude of 5-15 km above the ground. In the 60s, it was established that the force of the explosion was equal to the power of a hydrogen bomb with a capacity of 50 megatons.
Today, there are a huge number of assumptions and theories about the fall of this celestial body. The official verdict says that it was not a meteorite that fell to Earth, but a comet - a block of ice interspersed with tiny solid cosmic particles.
Some researchers believe that an alien spaceship crashed over our planet. In general, almost nothing is known about the Tunguska meteorite. No one can name the parameters and mass of this stellar body. The prospectors will probably never come to the only correct concept. After all, how many people, so many opinions. Therefore, the riddle of the Tungus guest will give birth to more and more new hypotheses.
Meteorite- this is a solid extraterrestrial substance that was preserved during the passage through the atmosphere and reached the surface of the Earth. Meteorites are the most primitive of the SS, which have not experienced further fractionation since their formation. This is based on the fact that the relative distribution refractory el. in meteorites corresponds to the solar distribution. Meteorites are classified into (according to the content of the metal phase): Stone(aeroliths): achondrites, chondrites, iron stone(siderolites), iron(siderites). Iron meteorites - consist of kamacite - native Fe of cosmic origin with an admixture of nickel from 6 to 9%. Iron stone meteorites Small distribution Group. They have coarse-grained structures with equal weight proportions of silicate and Fe phases. (Silicate minerals - Ol, Px; Fe phase - kamacite with Widmanstätten intergrowths). Stone meteorites - consist of silicates of Mg and Fe with an admixture of metals. Subdivided into Chondrite, achondrite and carbonaceous.Chondrites: spheroidal segregations of the first mm or less in size, composed of silicates, less often silicate glass. Embedded in a Fe-rich matrix. The groundmass of chondrites is a fine-grained mixture of Ol, Px (Ol-bronzite, Ol-hypersthene and Ol-pijonitic) with nickel Fe (Ni-4-7%), troilite (FeS) and plagioclase. Chondrites - crystallized. or glassy drops, cat. Image. when melting a pre-existing silicate material subjected to heating. Achondrites: Do not contain chondrules, have a lower content. nickel Fe and coarser structures. Their main minerals are Px and Pl, some types are enriched in Ol. Achondrites are similar in composition and structural features to terrestrial Gabbroids. The composition and structure speak of a magmatic origin. Sometimes there are bubbly structures like lavas. Carbonaceous chondrites (large amounts of carbonaceous matter) Characteristic feature of carbonaceous chondrites - the presence of a volatile component, which indicates primitiveness (the removal of volatile elements did not occur) and did not undergo fractionation. Type C1 contains a large number of chlorite(aqueous Mg, Fe aluminosilicates), as well as magnetite, water-soluble salt, nativeS, dolomite, olivine, graphite, organ. connections. Those. since their image-I they are noun. at T, not > 300 0 С. chondrite meteorites lack of 1/3 chem. Email compared to composition carbonaceous chondrites, cat. closest to the composition of protoplanetary matter. The most likely cause of the shortage of volatile email. - sequential condensation el. and their compounds in reverse order of their volatility.
5.Historical and modern models of accretion and differentiation of protoplanetary matter O.Yu. Schmidt in the 40s expressed the idea that the Earth and the planets of the CG were formed not from hot clots of solar gases, but through the accumulation of HB. bodies and particles - planetesimals that experienced melting later during accretion (heating due to collisions of large planetesimals, up to a few hundreds of kilometers in diameter). Those. early differentiation of the core and mantle and degassing. Ex. relates two points of view. accumulation mechanism and ideas about the form of the layered structure of the planets. Models homogeneous and heterogeneous accretion: HETEROGENEOUS ACCRETION 1. Short-term accretion. Early heterogeneous accretion models(Turekian, Vinogradov) assumed that Z. accumulated from the material as it condensed from the protoplanetary cloud. Early models include an early > T accumulation of the Fe-Ni alloy, which forms the proto-core of Z., changing from lower. T by accretion of its outer parts from silicates. Now it is believed that in the process of accretion there is a continuous change. in the accumulating material of the Fe/silicate ratio from the center to the periphery of the formed planet. As the earth accumulates, it heats up and melts Fe, which separates from the silicates and sinks into the core. After the cooling of the planet, about 20% of its mass is added with material enriched in volatiles along the periphery. In the proto-earth, there were no sharp boundaries between the core and the mantle, a cat. established as a result of gravitation. and chem. differentiation at the next stage of the evolution of the planet. In the early versions, differentiation occurred mainly during the formation of the ZK, and did not capture the Earth as a whole. HOMOGENEOUS ACCRETION 2. A longer accretion time of 108 years is assumed. During the accretion of the Earth and the planets of the Earth, the condensing bodies had wide variations in composition from carbonaceous chondrites enriched in volatiles to substances enriched in refractory components of the Allende type. Planets of forms. from this set of meteorites in-va and their difference and similarity was determined by relative. proportions in-va different composition. It also took place macroscopic homogeneity of protoplanets. The existence of a massive core indicates that the alloy originally introduced by Fe-Ni meteorites, uniformly distributed throughout the Earth, separated out in the course of its evolution into the central part. Homogeneous in composition the planet was stratified into shells in the process of gravitational differentiation and chemical processes. Modern model of heterogeneous accretion to explain the chem. the composition of the mantle is being developed by a group of German scientists (Wencke, Dreybus, Yagoutz). They found that the content in the mantle of moderately volatile (Na, K, Rb) and moderately siderophilic (Ni, Co) el., with different. The distribution coefficients of Me/silicate have the same abundance (normalized by C1) in the mantle, and the most strongly siderophile elements have excess concentrations. Those. the core was not in equilibrium with the mantle reservoir. They proposed heterogeneous accretion :one. Accretion begins with the accumulation of a strongly reduced component A, devoid of volatile elements. and containing all the other email. in quantities corresponding to C1, and Fe and all siderophiles in the reduced state. With an increase in T, the formation of a nucleus begins simultaneously with accretion. 2. After accretion, more and more oxidized material, component B, begins to accumulate in 2/3 of the earth's mass. and transfer them to the kernel. A source of moderately volatile, volatile and moderately siderophilic el. in the mantle yavl. component B, which explains their close relative abundance. Thus, the Earth is 85% composed of component A and 15% of component B. In general, the composition of the mantle is formed after separation of the core by homogenization and mixing of the silicate part of component A and the substance of component B.
6. Isotopes of chemical elements. isotopes - atoms of the same electron, but having a different number of neutrons N. They differ only in mass. isotons - atoms of different el., having different Z, but the same N. They are arranged in vertical rows. isobars - atoms of different el., in a cat. equal masses. numbers (A=A), but different Z and N. They are arranged in diagonal rows. Nuclear stability and isotope abundance; radionuclides The number of known nuclides is ~ 1700, of which ~ 260 are stable. On the nuclide diagram, stable isotopes (shaded squares) form a band surrounded by unstable nuclides. Only nuclides with a certain ratio of Z and N are stable. The ratio of N to Z increases from 1 to ~ 3 with increasing A. 1. Nuclides are stable, in a cat. N and Z are approximately equal. Up to Ca in N=Z nuclei. 2. Most stable nuclides have even Z and N. 3. Less common are stable nuclides with even numbers. Z and odd. N or even N and odd. Z. 4. Rare stable nuclides with odd Z and N.
|
number of stable nuclides | ||||
|
odd |
odd | |||
|
odd |
odd | |||
|
odd |
odd |
In kernels from even. Z and N nucleons form an ordered structure, which determines their stability. The number of isotopes is less in light email. and took away. in the middle part of the PS, reaching a maximum for Sn (Z=50), which has 10 stable isotopes. Elements with odd. Z stable isotopes no more than 2.
7. Radioactivity and its types Radioactivity - spontaneous transformations of the nuclei of unstable atoms (radionuclides) into stable nuclei of other elements, accompanied by emission of particles and/or radiation of energy. St. glad-ty does not depend on the chemical. Holy atoms, but determined by the structure of their nuclei. Radioactive decay is accompanied by changes. Z and N of the parent atom and leads to the transformation of an atom of one el. into an atom of another email. It has also been shown by Rutherford and other scientists that he is glad. the decay is accompanied by the emission of radiation of three different types, a, b, g. a-rays - streams of high-speed particles - He nuclei, b - rays - streams e - , g - rays - electromagnetic waves with high energy and shorter λ. Types of radioactivity a-decay- decay by emission of a-particles, it is possible for nuclides with Z> 58 (Ce), and for a group of nuclides with small Z, including 5He, 5Li, 6Be. a-particle consists of 2 P and 2N, there is a shift of 2 positions in Z. The initial isotope is called parental or maternal, and the newly formed - child.
b-decay- has three types: normal b-decay, positron b-decay and e - capture. Ordinary b-decay- can be considered as the transformation of a neutron into a proton and e - , the last or beta particle - is ejected from the nucleus, accompanied by the emission of energy in the form of g-radiation. The daughter nuclide is an isobar of the parent, but its charge is greater.
There is a series of decays until a stable nuclide is formed. Example: 19 K40 -> 20 Ca40 b - v - Q. Positron b-decay- emission from the nucleus of a positive particle of a positron b, its formation - the transformation of a nuclear proton into a neutron, positron and neutrino. The daughter nuclide is an isobar but has a smaller charge.
Example, 9 F18 -> 8 O18 b v Q while the number N decreases. Atoms to the left of the region of nuclear stability are neutron-deficient, they undergo positron decay, and their number N increases. Thus, during b- and b-decay, there is a tendency for Z and N to change, leading to the approach of daughter nuclides to the zone of nuclear stability. e – capture- capture of one of the orbital electrons. High probability of capture from the K-shell, cat. closest to the core. e - capture causes emission from the neutrino nucleus. Daughter nuclide yavl. isobar, and occupies the same position relative to the parent as in positron decay. b - radiation is absent, and when a vacancy is filled in the K-shell, X-rays are emitted. At g radiation neither Z nor A change; when the nucleus returns to its normal state, energy is released in the form g-radiation. Some daughter nuclides of the natural isotopes U and Th can decay either by emitting b-particles or by a-decay. If b-decay occurred first, then a-decay followed, and vice versa. In other words, these two alternative decay modes form closed cycles and always lead to the same end product - stable isotopes of Pb.
8. Geochemical consequences of the radioactivity of terrestrial matter. Lord Kelvin (William Thomson) from 1862 to 1899 performed a series of calculations, cat. imposed restrictions on the possible age of the Earth. They were based on consideration of the luminosity of the Sun, the influence of lunar tides, and the processes of cooling of the Earth. He came to the conclusion that the age of the Earth is 20-40 million years. Later, Rutherford performed the determination of the age of U min. and received values of about 500 million years. Later, Arthur Holmes in his book "The Age of the Earth" (1913) showed the importance of the study of radioactivity in geochronology and gave the first GHS. It was based on consideration of data on the thickness of sedimentary deposits and on the content of radiogenic decay products - He and Pb in U-bearing minerals. Geological scale- the scale of the natural historical development of the ZK, expressed in numerical units of time. The accretion age of Earth is about 4.55 billion years. The period up to 4 or 3.8 billion years is the time of differentiation of the planetary interior and the formation of the primary crust, it is called katarchey. The longest period of life of Z. and ZK is the Precambrian, cat. extends from 4 billion years to 570 million years, i.e. about 3.5 billion years. The age of the most ancient rocks known now exceeds 4 billion years.
9. Geochemical classification of elements by V.M. HolshmidtBased on: 1- distribution email. between different phases of meteorites - separation during the primary HX differentiation of Z. 2 - specific chemical affinity with certain elements (O, S, Fe), 3 - structure of electron shells. The leading elements that make up meteorites are O, Fe, Mg, Si, S. Meteorites consist of three main phases: 1) metal, 2) sulfide, 3) silicate. All e-mail are distributed between these three phases in accordance with their relative affinity for O, Fe and S. In the Goldschmidt classification, the following groups of elec. are distinguished: 1) siderophilic(loving iron) - metal. phase of meteorites: el., forming alloys of arbitrary composition with Fe - Fe, Co, Ni, all platinoids (Ru, Rh, Pd, Pt, Re, Os, Ir), and Mo. They often have a native state. These are transitional elements of group VIII and some of their neighbors. Form the inner core Z. 2) Chalcophilic(copper-loving) - the sulfide phase of meteorites: elements that form natural compounds with S and its analogues Se and Te also have an affinity for As (arsenic), sometimes they are called (sulfurophilic). Easily pass into a native state. These are elements of secondary subgroups I-II and main subgroups III-VI groups of PS from 4 to 6 period S. The most famous are Cu, Zn, Pb, Hg, Sn, Bi, Au, Ag. Siderophile el. – Ni, Co, Mo can also be chalcophilic with a large amount of S. Fe under reducing conditions has an affinity for S (FeS2). In the modern model of the star, these metals form the outer, sulfur-enriched core of the star.
3) lithophilic(loving stone) - silicate phase of meteorites: el., having an affinity for O 2 (oxyphilic). They form oxygen compounds - oxides, hydroxides, salts of oxygen acids-silicates. In compounds with oxygen, they have an 8-electron ext. shell. This is the largest group of 54 elements (C, widespread petrogenic - Si, Al, Mg, Ca, Na, K, elements of the iron family - Ti, V, Cr, Mn, rare - Li, Be, B, Rb, Cs, Sr , Ba, Zr, Nb, Ta, REE, i.e. all the rest except atmophilic ones). Under oxidizing conditions, iron is oxyphilic - Fe2O3. form the mantle Z. 4) Atmophilic(har-but gaseous state) - chondrite matrix: H, N inert gases (He, Ne, Ar, Kr, Xe, Rn). They form the atmosphere Z. There are also such groups: rare earth Y, alkaline, large-ion lithophile elements LILE (K, Rb, Cs, Ba, Sr), high-charge elements or elements with high field strength HFSE (Ti, Zr, Hf, Nb, Ta , Th). Some definitions of email: petrogenic (rock-forming, main) minor, rare, trace elements- with conc. no more than 0.01%. scattered- microel. not forming their own minerals accessory- form accessory min. ore- form ore mines.
10. The main properties of atoms and ions that determine their behavior in natural systems. Orbital radii - radii of the maxima of the radial density e – ext. orbitals. They reflect the sizes of atoms or ions in the free state, i.e. outside the chem. connections. The main factor is e - the structure of the electron, and the more e - shells, the larger the size. For def. sizes of atoms or ions in an important way yavl. Def. distance from the center of one atom to the center of another, cat. is called the bond length. For this, X-ray methods are used. In the first approximation, atoms are considered as spheres, and the “principle of additivity” is applied, i.e. it is believed that the interatomic distance is the sum of the radii of the atoms or ions that make up the in-in. Then knowing or accepting a certain value as the radius of one el. you can calculate the dimensions of all others. The radius calculated in this way is called effective radius . coordination number is the number of atoms or ions located in close proximity around the considered atom or ion. CF is determined by the ratio R k /R a: Valence - the amount of e - given or attached to the atom during the formation of chemical. connections. Ionization potential is the energy required to remove e- from an atom. It depends on the structure of the atom and is determined experimentally. The ionization potential corresponds to the voltage of the cathode rays, which is sufficient to ionize an atom of this email. There may be several ionization potentials, for several e - removed from the external. e - shells. The separation of each subsequent e - requires more energy and may not always be. Usually use the ionization potential of the 1st e - , cat. detects periodicity. On the curve of ionization potentials, alkali metals, which easily lose e - , occupy minima on the curve, inert gases - peaks. As the atomic number increases, the ionization potentials increase in the period and decrease in the group. The reciprocal is the affinity ke – . Electronegativity - the ability to attract e - when entering into compounds. The halogens are the most electronegative, the alkali metals the least. Electronegativity depends on the charge of the nucleus of an atom, its valency in a given compound, and the structure of the e-shells. Repeated attempts have been made to express EC in units of energy or in conventional units. The EC values regularly change by groups and periods of PS. EO is minimal for alkali metals and increases for halogens. In lithophilic cations, EO is reduced. from Li to Cs and from Mg to Ba, i.e. with a zoom ionic radius. In chalcophile el. EO is higher than that of lithophiles from the same PS group. For anions of the O and F groups, the EO decreases down the group and, therefore, it is maximum for these el. Email with sharply different EO values form compounds with an ionic type of bond, and with close and high - with a covalent, with close and low - with a metallic type of bond. The ionic potential of Cartledge (I) is equal to the ratio of valency to R i , it reflects the properties of cationicity or ionogenicity. V.M. Golshmidt showed that the properties of cationicity and anionicity depend on the ratio of valence (W) and R i for ions of the noble gas type. In 1928, K. Cartledge called this ratio the ionic potential I. At small values of I el. behaves like a typical metal and cation (alkali and alkaline earth metals), and at large - like a typical non-metal and anion (halogens). These relationships are conveniently depicted graphically. Diagram: ionic radius - valency. The value of the ionic potential allows us to judge the mobility of email. in the aquatic environment. Email with low and high values of I are the most mobile easily (with low values they pass into ionic solutions and migrate, with high values they form complex soluble ions and migrate), and with intermediate ones they are inert. The main types of chem. bonds, character bonds in the main groups of minerals. Ionic- image due to the attraction of ions with opposite charges. (with a large difference in electronegativity) Ionic bonding predominates in most mines. ZK - oxides and silicates, this is the most common type of bond also in hydro and atmospheres. Communication provides easy dissociation of ions in melts, solutions, gases, due to which there is a wide migration of chemical. El., their dispersion and end in the terrestrial geospheres. covalent - noun. due to the interaction e - used by different atoms. Typical for e. with an equal degree of attraction e – , i.e. EO. Har-na for liquid and gaseous substances (H2O, H2, O2, N2) and less for a crystal. Sulfides, related compounds As, Sb, Te, as well as monoel are characterized by a covalent bond. non-metal compounds - graphite, diamond. Covalent compounds are characterized by low solubility. metal- a special case of a covalent bond, when each atom shares its e - with all neighboring atoms. e - capable of free movement. Typical for native metals (Cu, Fe, Ag, Au, Pt). Many min. have a connection, a cat. partly ionic, partly covalent. in sulfide mines. the covalent bond is maximally manifested, it takes place between the metal and S atoms, and the metallic one - between the metal atoms (metal, brilliance of sulfides). Polarization - this is the effect of distortion of the e-cloud of an anion by a small cation with a large valence so that a small cation, attracting a large anion to itself, reduces its effective R, itself entering its e-cloud. So the cation and anion are not regular spheres, and the cation causes the deformation of the anion. The higher the charge of the cation and the smaller its size, the stronger the effect of polarization. And the larger the size of the anion and its negative charge, the stronger it is polarized - deformed. Lithophilic cations (with 8 electron shells) cause less polarization than ions with completed shells (like Fe). Chalcophile ions with large serial numbers and high-valent cause the strongest polarization. This is associated with the formation of complex compounds: 2-, , 2-, 2-, cat. soluble and yavl. the main carriers of metals in hydrothermal solutions.
11.Status (form of location) email. in nature. In GC allocate: actually min. (crystal. phases), impurities in min., various forms of the scattered state; email location form in nature carries information about the degree of ionization, har-re chem. email connections in phases, etc. V-in (el.) is in three main forms. The first is the end atoms, the image. stars are different. types, gaseous nebulae, planets, comets, meteorites and space. tv. particles in-va. Degree of conc. V-va in all bodies is different. The most scattered states of atoms in gaseous nebulae are held by gravitational forces or are on the verge of overcoming them. The second - scattered atoms and molecules, an image of interstellar and intergalactic gas, consisting of free atoms, ions, molecules, e -. Its quantity in our Galaxy is much less than that which is concentrated in stars and gaseous nebulae. Interstellar gas is located at different sparse stages. The third one is intensively migrating atomic nuclei and elementary particles flying at a tremendous speed, which make up cosmic rays. IN AND. Vernadsky singled out the main four forms of finding chem. Email in the ZK and on its surface: 1. rocks and minerals (solid crystalline phases), 2. magmas, 3. scattered state, 4. living matter. Each of these forms is distinguished by the special state of their atoms. Ex. and other allocation of forms of finding e-mail. in nature, depending on the specific sv-in themselves email. A.I. Perelman singled out mobile and inert forms finding chem. Email in the lithosphere. By his definition, movable form is such a state of chemistry. Email in gp, soils and ores, being in a cat. Email can easily pass into the solution and migrate. inert form represents such a state in urban settlements, ores, weathering crust and soils, in the cat. Email under the conditions of this situation, it has a low migratory mode and cannot move into the solution and migrate.
12. Internal factors of migration.
Migration- movement of chemicals Email in geospheres Z, leading to their dispersion or conc. Clarke - medium conc. in the main types of GP ZK of each chem. Email can be considered as a state of its equilibrium under the conditions of a given chemical. Wednesdays, a deviation from a cat. gradually reduced by migrating this email. Under terrestrial conditions, the migration of chemical Email happens in any medium - TV. and gaseous (diffusion), but easier in a liquid medium (in melts and aqueous solutions). At the same time, the forms of migration of chemical Email are also different - they can migrate in atomic (gases, melts), ionic (solutions, melts), molecular (gases, solutions, melts), colloidal (solutions) forms and, in the form of detrital particles (air and water environment ). A.I. Perelman distinguishes four types of chemical migration. El.: 1.mechanical, 2.phys.-chemical, 3.biogenic, 4.technogenic. The most important internal factors: 1. Thermal properties of electricity, i.e. their volatility or infusibility. El., having a condensation T of more than 1400 o K are called refractory platinoids, lithophilic - Ca, Al, Ti, Ree, Zr, Ba, Sr, U, Th), from 1400 to 670 o K - moderately volatile. [lithophile - Mg, Si (moderately refractory), many chalcophile, siderophile - Fe, Ni, Co],< 670 o K – летучими (атмофильные). На основании этих св-в произошло разделение эл. по геосферам З. При магм. процессе в условиях высоких Т способность к миграции будет зависеть от возможности образования тугооплавких соединений и, нахождения в твердой фазе. 2. Хим. Св-ва эл. и их соединений. Атомы и ионы, обладающие слишком большими или слишком малыми R или q, обладают и повышенной способностью к миграции и перераспределению. Хим. Св-ва эл. и их соединений приобретают все большее значение по мере снижения T при миграции в водной среде. Для литофильных эл. с низким ионным потенциалом (Na, Ca, Mg) в р-рах хар-ны ионные соединения, обладающие высокой раствор-ю и высокими миграционными способностями. Эл. с высокими ионными потенциалами образуют растворимые комплексные анионы (С, S, N, B). При низких Т высокие миграционные способности газов обеспечиваются слабыми молекулярными связями их молекул. Рад. Св-ва, опред-ие изменение изотопного состава и появление ядер других эл.
Meteorites are cosmic bodies falling to Earth from the 2nd space. speed, therefore, they experience heating, melting, explosion The surface of the planets has a characteristic appearance of collisions
Types of meteorites: 1) Stone - Ch. MgFe silicate components, metal impurities. 2) Iron - Fe + Ni alloy. 3) Iron stone - intermediate. meteorite minerals(main components): 1) Silicates (olivine, pyroxene). 2) Plagioclase is rare. 3) Layered silicates (with water - serpentine, chlorite) - extremely rare. 4) Metallic iron (tennessite and kamacite) differ in Ni content. 5) sulfide FeS - troilite (not common): (on average, meteorites - y / o substance). Apatite, magnetite diamond, lonsdaleite are important for understanding the genesis - MgS (MgS-FeS) CaS (oltgamite) indicate oxygen deficiency during formation. Carbides - FeC, MgC. TiN nitrides. The problem of chemistry is complex - the proportions are violated: Stone - kg, (destroyed in the atmosphere), iron - tens of thousands of tons. Meteorites-finds meteorites-falls. -Statistics of finds - iron ones predominate. - Fall statistics - stone
7. Chondrites. Formation of the planets of the solar system
Stone. The main type of M. is stone, among them 90% are chondrites. Chondrules - density 3, formation not in planetary gravitational fields. The balls indicate the formation in a liquid state, the crystallization structure is quenching. Structure - Olivine (skeletal crystals), pyroxene (quenching). Chondrules are the result of a rapid cooling of a silicate substance in unknown processes (multiple evaporation and condensation). The substance has not passed the planetary stage of development. Chondrite types: Enstatite chondrite MgSiO3 + Fe itself. (met. phase) - restoration of the situation. Carbonaceous chondrites - no native Fe, there is magnetite. C carbon - up to 2-3%, C H2O - the first% (Sp, chl).
Meteorites-finds meteorites-falls. - Primary substance? - Enriched with volatile components. Achondrites (devoid of chondrite structure). - As a result of fur deformations (collisions), diamonds appear. - Brecciated (fragments of chondrules). -Basaltoids (pyroxene plagioclase olivine) of other origin, (there are few of them).
Iron meteorites: Tennessite + kamacite. The structure is lamellar, lattice - kamacite beams. Windmanstetten structure hardening temperature 600 °C. Important - such structures could not be repeated in laboratory conditions (Fe condensation), the same structure of iron in interstitium in chondrites
Troilite nodules. - a rare admixture of silicates. - Iron-stony meteorites: - Pallasites - uniform mixture without differentiation into light and heavy phases. -Their role is very small. -The history of meteorites is captured in isotopic composition. - It turned out that the substance is ancient - 4.55 * 10 * 9 years. -This is the age of the Earth, the Moon and meteoric matter. - "cosmic age" of meteorites of 100-200 million years is determined by short-lived isotopes formed on the surface of M. under the influence of cosmic radiation. -That is, meteorites are young formations that arose as a result of the crushing of space. tel
The abundance of elements in meteorites: The main position developed by Goldschmit on chondrites. The identity of the abundance of elements in chondrites and in the solar system. The abundance of elements in meteorites: It is reasonably believed that chondrites are undifferentiated primary matter. But there are also differences from the solar system: 1. H and inert gases are very rare in meteorites. 2. Depleted in Pb, Ge, Cd, Bi, Hg, but not as much as in inert gases. That is, chondrites are only a solid fraction of the primary substance (without a volatile substance). The composition of the terrestrial planets is associated with this fraction. The main process of planet formation is the condensation of a gas-dust cloud.
8. Patterns of the structure of the terrestrial planets
Planets differ in size, density, mass, distance from the Sun, and other parameters. They are divided into two groups: internal (Mercury, Venus, Earth, Mars) and external (Jupiter, Saturn, Uranus, Neptune). They are separated by a ring of asteroids between Mars and Jupiter. As they move away from the Sun, the planets, up to the Earth, increase and become more dense (3.3–3.5 g / cm3), and the outer planets decrease, starting from Jupiter, and less dense (0.71–2.00 g /cm3). In the inner planets, a silicate and a metallic phase are distinguished, the latter is expressed in Mercury (62%). The closer a planet is to the Sun, the more iron it contains. The outer planets are composed of gas components (H, He, CH4, NH3, etc.). The planets have one or more satellites, with the exception of Mercury and Venus.
9. Surface shells of planets
planetary shells. P.'s structure along the vertical is layered, several are distinguished. spherical shells, differing in chemical. composition, phase state, density, etc. physical-chemical. characteristics. All planets of the terrestrial group have hard shells, in which almost all of their mass is concentrated. Three of them - Venus, Earth and Mars - have gaseous atmospheres, Mercury is practically devoid of an atmosphere. Only the Earth has a liquid shell (discontinuous) of water - the hydrosphere, as well as the biosphere - the shell, composition, structure and energy of a cut in essential terms are due to the past and modern. activities of living organisms. An analogue of the hydrosphere on Mars is yavl. cryosphere - H 2 O ice in the polar caps and in the ground (permafrost). One of the mysteries of the solar system is the scarcity of water on Venus. There is no liquid water there because of the high temperature, and the amount of water vapor in the atmosphere is equivalent to a liquid layer ≈ 1 cm thick. equilibrium, since the yield strength of rocks corresponds to the weight of a column of rocks ≈10 km high (for the Earth). Therefore, the shape of the hard shells of P., which have a much greater thickness, is almost spherical. Due to the difference in gravity force different max. the height of the mountains on P. (for example, on Earth, about 10 km, and on Mars, where the gravitational field is weaker than the earth's, about 25 km). The shape of small satellites of planets and asteroids can differ markedly from spherical.
10. Origin of earthly shells
The geographical shell is formed by two fundamentally different types of matter: atomic-molecular "non-living" matter and atomic-organismic "living" matter. The first can participate only in physicochemical processes, as a result of which new substances can appear, but from the same chemical elements. The second has the ability to reproduce its own kind, but of a different composition and appearance. Interactions of the former require external energy costs, while the latter have their own energy and can give it away during various interactions. Both types of matter arose simultaneously and have been functioning since the beginning of the formation of the terrestrial spheres. Between parts of the geographic shell, there is a constant exchange of matter and energy, which manifests itself in the form of atmospheric and oceanic circulation, the movement of surface and ground waters, glaciers, the movement of organisms and living matter, etc. Due to the movement of matter and energy, all parts of the geographic shell are interconnected and form an integral system
11. The structure and composition of the earth's shells
The lithosphere, atmosphere and hydrosphere form practically continuous shells. The biosphere as a set of living organisms in a certain habitat does not occupy an independent space, but masters the above-mentioned spheres completely (hydrosphere) or partially (atmosphere and lithosphere).
The geographical envelope is characterized by the allocation of zonal-provincial isolations, which are called landscapes, or geosystems. These complexes arise with a certain interaction and integration of geocomponents. The simplest geosystems are formed by the interaction of matter at the inert level of organization.
Chemical elements in the geographic shell are in a free state (in air), in the form of ions (in water) and complex compounds (living organisms, minerals, etc.).
12. Structure and composition of the mantle
Mantle- part of the Earth (geosphere), located directly under the crust and above the core. The mantle contains most of the Earth's matter. The mantle is also found on other planets. The earth's mantle is in the range from 30 to 2900 km from the earth's surface.
The boundary between the crust and the mantle is the Mohorovichic boundary, or Moho for short. There is a sharp increase in seismic velocities on it - from 7 to 8-8.2 km / s. This border is located at a depth of 7 (under the oceans) to 70 kilometers (under the fold belts). The Earth's mantle is divided into the upper mantle and the lower mantle. The boundary between these geospheres is the Golitsyn layer, located at a depth of about 670 km.
The difference in the composition of the earth's crust and mantle is a consequence of their origin: the initially homogeneous Earth, as a result of partial melting, was divided into a fusible and light part - the crust and a dense and refractory mantle.
The mantle is composed mainly of ultrabasic rocks: perovskites, peridotites (lherzolites, harzburgites, wehrlites, pyroxenites), dunites and, to a lesser extent, basic rocks - eclogites.
Also, among the mantle rocks, rare varieties of rocks that are not found in the earth's crust have been identified. These are various phlogopite peridotites, grospidites, and carbonatites.
The structure of the mantle
The processes taking place in the mantle have the most direct impact on the earth's crust and surface of the earth, are the cause of the movement of continents, volcanism, earthquakes, mountain building and the formation of ore deposits. There is growing evidence that the mantle itself is actively influenced by the Earth's metallic core.
13. The structure and composition of the earth's crust
The structure of the globe. The main object of geological, including mineralogical, research is Earth's crust*, which means the uppermost shell of the globe, accessible to direct observation. These include: the lower part of the atmosphere, the hydrosphere and the upper part of the lithosphere, that is, the solid part of the Earth.
The hypothesis of V. M. Goldshmidt about the structure of the globe is currently enjoying the greatest recognition. The latter, according to his ideas, consists of three main concentrically located zones (geospheres):
outer - lithosphere;
intermediate - chalcosphere, rich in oxides and sulfur compounds of metals, mainly iron,
central - siderosphere, represented by an iron-nickel core.
The lithosphere, in turn, is divided into two parts:
the upper shell - up to a depth of 120 km, composed mainly of ordinary silicate rocks,
the lower one is an eclogitic shell (120-1200 km), represented by silicate rocks enriched in magnesium.
The composition of the earth's crust.
The most common elements are: O, Si, Al, Fe, Ca, Na, K, Mg, H, Ti, C and Cl. The remaining 80 elements account for only 0.71% (by weight)
Iron meteorites represent the largest group of meteorite finds outside of the hot deserts of Africa and the ice of Antarctica, as non-specialists can easily identify them by their metallic composition and large weight. In addition, they weather more slowly than stone meteorites and, as a rule, are much larger due to their high density and strength, which prevent their destruction when passing through the atmosphere and falling to the ground. Despite this fact, as well as the fact that iron meteorites have a common weighing more than 300 tons accounts for more than 80% of the total mass of all known meteorites, they are relatively rare. Iron meteorites are often found and identified, but they account for only 5.7% of all observed falls. From the point of view of classification, iron meteorites are divided into groups according to two completely different principles. The first principle is a kind of relic of classical meteoritics and involves the division of iron meteorites according to structure and dominant mineral composition, and the second is a modern attempt to divide meteorites into chemical classes and correlate them with certain parent bodies. Structural classification Iron meteorites mainly consist of two iron-nickel minerals - kamazite with a nickel content of up to 7.5% and taenite with a nickel content of 27% to 65%. Iron meteorites have a specific structure, depending on the content and distribution of one or another mineral, on the basis of which classical meteoritics divides them into three structural classes. OctahedritesHexahedritesAtaxitesOctahedrites
Octahedrites consist of two metal phases - kamacite (93.1% iron, 6.7% nickel, 0.2 cobalt) and taenite (75.3% iron, 24.4% nickel, 0.3 cobalt) which form a three-dimensional octahedral structures. If such a meteorite is polished and its surface treated with nitric acid, the so-called Widmanstatt structure appears on the surface, a delightful play of geometric shapes. These groups of meteorites differ depending on the width of the camasite bands: coarse-grained nickel-poor broadband octahedrites with a band width of more than 1.3 mm, medium octahedrites with a band width of 0.5 to 1.3 mm, and fine-grained nickel-rich octahedrites with a band width less than 0.5 mm. Hexahedrites Hexahedrites are composed almost entirely of nickel-poor kamazite and, when polished and etched, do not reveal the Widmanstätten structure. In many hexahedrites, after etching, thin parallel lines appear, the so-called Neumann lines, reflecting the structure of kamazite and, possibly, being a consequence of impact, the collision of the parent body of hexahedrites with another meteorite. Ataxites After etching, ataxites show no structure, but, unlike hexahedrites, they are composed almost entirely of taenite and contain only microscopic lamellae of kamazite. They are among the richest in nickel (the content of which exceeds 16%), but also the rarest meteorites. However, the world of meteorites is an amazing world: paradoxically, the largest meteorite on Earth, the Goba meteorite from Namibia, weighing over 60 tons, belongs to the rare class of ataxites.
Chemical classification In addition to the content of iron and nickel, meteorites differ in the content of other minerals, as well as the presence of traces of rare earth metals such as germanium, gallium, iridium. Studies of the ratio of metal trace elements and nickel have shown the presence of certain chemical groups of iron meteorites, and each of them is considered to correspond to a specific parent body. Here we briefly touch on thirteen established chemical groups, and it should be noted that about 15% of known iron meteorites do not fall into them meteorites, which are unique in their chemical composition. Compared to the Earth's iron-nickel core, most iron meteorites represent the cores of differentiated asteroids or planetoids that must have been destroyed by catastrophic impact before falling back to Earth as meteorites! Chemical groups:IABICIIABIICIIDIIEIIFIIIABIIICDIIIEIIIFIVAIVBUNGRIAB Group A significant part of iron meteorites belongs to this group, in which all structural classes are represented. Especially often among the meteorites of this group there are large and medium octahedrites, as well as iron meteorites rich in silicates, i.e. containing more or less large inclusions of various silicates chemically closely related to winonaites, a rare group of primitive achondrites. Therefore, both groups are considered to be descended from the same parent body. Often, IAB group meteorites contain inclusions of bronze-colored iron sulfide troilite and black graphite grains. Not only the presence of these rudimentary forms of carbon indicates a close relationship of the IAB group with Carboniferous chondrites; This conclusion also allows us to draw the distribution of microelements. IC group The much rarer iron meteorites of the IC group are very similar to the IAB group, with the difference that they contain less rare earth trace elements. Structurally, they belong to coarse-grained octahedrites, although iron meteorites of the IC group are also known, which have a different structure. Typical for this group is the frequent presence of dark inclusions of cementite cohenite in the absence of silicate inclusions. Group IIAB The meteorites of this group are hexahedrites, i.e. consist of very large individual crystals of kamazite. The distribution of trace elements in iron meteorites of the IIAB group resembles their distribution in some Carboniferous chondrites and enstatite chondrites, from which it can be concluded that the iron meteorites of the IIAB group originate from the same parent body. Group IIC Group IIC iron meteorites include the finest-grained octahedrites with kamazite bands less than 0.2 mm wide. The so-called “filling” plessite, a product of a particularly fine synthesis of taenite and kamazite, which also occurs in other octahedrites in a transitional form between taenite and kamazite, is the basis of the mineral composition of group IIC iron meteorites. Group III The meteorites of this group occupy a middle position at the transition to fine-grained octahedrites, distinguished by a similar distribution of trace elements and a very high content of gallium and germanium. Most Group IID meteorites contain numerous inclusions of iron-nickel phosphate, schreibersite, an extremely hard mineral that often makes cutting IID iron meteorites difficult. Group II Structurally, group IIE iron meteorites belong to the class of medium-grained octahedrites and often contain numerous inclusions of various iron-rich silicates. At the same time, unlike meteorites of the IAB group, silicate inclusions do not have the form of differentiated fragments, but of hardened, often clearly defined drops, which give the iron meteorites of the IIE group optical attractiveness. Chemically, group IIE meteorites are closely related to H-chondrites; it is possible that both groups of meteorites come from the same parent body. IIF group This small group includes plessitic octahedrites and ataxites, which have a high content of nickel, as well as a very high content of trace elements such as germanium and gallium. There is a certain chemical similarity with both Eagle group pallasite and CO and CV group Carboniferous chondrites. Possibly, the pallasites of the “Eagle” group originate from the same parent body. Group IIIAB After the IAB group, the most numerous group of iron meteorites is the IIIAB group. Structurally, they belong to coarse and medium-grained octahedrites. Sometimes inclusions of troilite and graphite are found in these meteorites, while silicate inclusions are extremely rare. However, there are similarities with the main group Pallasites, and today both groups are thought to be descended from the same parent body.
Group IIICD Structurally, the IIICD group meteorites are the finest-grained octahedrites and ataxites, and in chemical composition they are closely related to the IAB group meteorites. Like the latter, Group IIICD iron meteorites often contain silicate inclusions, and today both groups are thought to have come from the same parent body. As a consequence, they also bear a resemblance to the Winonaites, a rare group of primitive achondrites. For iron meteorites of the IIICD group, the presence of a rare mineral hexonite (Fe,Ni) 23 C 6 is typical, which is present exclusively in meteorites. Group IIIE Structurally and chemically, iron meteorites of group IIIE are very similar to meteorites of group IIIAB, differing from them in a unique distribution of trace elements and typical hexonite inclusions, which makes them similar to meteorites of group IIICD. Therefore, it is not entirely clear whether they form an independent group derived from a separate parent body. Perhaps further research will provide an answer to this question. Group IIIF Structurally, this small group includes coarse-grained to fine-grained octahedrites, but differs from other iron meteorites in both relatively low nickel content and very low abundance and unique distribution of some trace elements. IVA group Structurally, IVA group meteorites belong to the class of fine-grained octahedrites and are distinguished by a unique distribution of trace elements. They have inclusions of troilite and graphite, while silicate inclusions are extremely rare. The only notable exception is the anomalous Steinbach meteorite, a historic German find, as it is almost half reddish-brown pyroxene in an iron-nickel IVA matrix. The question of whether it is the product of an impact on the IVA parent body or a relative of pallasite and, therefore, a stony iron meteorite is currently being vigorously discussed. Group IVB
All iron meteorites of the IVB group have a high nickel content (about 17%) and structurally belong to the class of ataxites. However, when observed under a microscope, one can see that they do not consist of pure taenite, but rather have a plessitic nature, i.e. were formed due to the fine synthesis of kamacite and taenite. A typical example of group IVB meteorites is Goba from Namibia, the largest meteorite on Earth. UNGR Group This abbreviation, meaning "not included in the group", denotes all meteorites that cannot be assigned to the above chemical groups. Although researchers currently classify these meteorites into twenty different small groups, recognition of a new meteorite group generally requires at least five meteorites, as established by the International Nomenclature Committee of the Meteor Society. The presence of this requirement prevents the hasty recognition of new groups, which in the future turn out to be only an offshoot of another group.