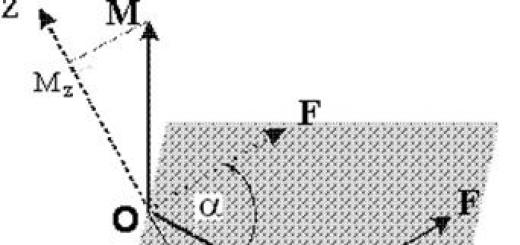
For the first time in the world, scientists have managed to obtain a visual image of a molecule in the resolution of single atoms in the process of rearranging its molecular bonds. The resulting image turned out to be surprisingly similar to pictures from chemistry textbooks.

Until now, scientists could only draw hypothetical conclusions about molecular structures. But with the help of new technology, the individual atomic bonds - each a few ten-millionths of a millimeter long - connecting the 26 carbon atoms and 14 hydrogen atoms in this molecule become clearly visible. The results of this study were published May 30 in the journal Science.
The team of experimenters initially aimed to precisely assemble nanostructures from graphene, a single-layer atomic material in which carbon atoms are arranged in a repeating hexagonal pattern. Creating a carbon honeycomb requires rearrangement of atoms from a linear chain to a hexagonal network; such a reaction can create several different molecules. Berkeley chemist Felix Fischer and his colleagues wanted to visualize the molecules to make sure they were doing everything right.

The carbon-containing molecule in the photo is shown before and after its rearrangement, with the inclusion of two of the most common reaction products. Image scale - 3 angstroms, or 3 ten-billionths of a meter
To document the graphene recipe, Fisher needed a very powerful optical instrument, and he used an atomic microscope located in a laboratory at the University of Berkeley. Non-contact atomic microscopes use an extremely sensitive needle to read the electrical forces produced by molecules; as the tip of the needle moves along the surface of the molecule, it is deflected by various charges, creating an image of how the atoms are arranged and the bonds between them.
With its help, the team of researchers managed not only to visualize carbon atoms, but also the bonds created by electrons between them. They placed a ring-shaped molecule on a silver surface and heated it to change its shape. Subsequent cooling managed to fix the reaction products, among which were three unexpected components and one molecule that scientists expected.
Until now, scientists could only assume the presence of molecular structures. Today, with the help of atomic force microscopy, the individual atomic bonds (each a few tens of millionths of a millimeter long) connecting a molecule (26 carbon atoms and 14 hydrogen atoms) can be seen quite clearly.
Initially, the team wanted to work with structures made from graphene, a single-layer material in which carbon atoms are arranged in hexagons. Forming honeycombs of carbon, the atoms are rearranged from a linear chain into hexagons; this reaction can produce several different molecules.
Felix Fischer, a chemist at the University of California at Berkeley, and his colleagues wanted to visualize the molecules to make sure they got it right.
A ringed, carbon-containing molecule, shown before and after reorganization with the two most common reaction products at temperatures above 90 degrees Celsius. Size: 3 angstroms or three to ten billionths of a meter across.

In order to document the graphene recipe, Fisher needed a powerful imaging device and turned to an atomic force microscope that Michael Crommie of the University of California lab had.
Non-contact atomic force microscopy (NC-AFM) uses a very thin and sensitive sensor to sense the electrical force generated by molecules. The tip moves near the surface of the molecule, being deflected by different charges, creating an image of how the atoms move.
The single-atom tip of a non-contact atomic force microscope "probes" the surface with a sharp needle. The needle moves along the surface of the object under study, just as the phonograph needle passes through the grooves of a record. In addition to atoms, it is possible to "probe" atomic bonds

So the team managed not only to visualize carbon atoms, but also the bonds between them created by shared electrons. They placed carbon ring structures on a silver plate and heated it to reorganize the molecule. The refrigerated reaction products contained three unexpected products and only one molecule expected by scientists.
Hydrogen atom capturing electron clouds. And although modern physicists can even determine the shape of a proton with the help of accelerators, the hydrogen atom, apparently, will remain the smallest object, the image of which makes sense to call a photograph. "Lenta.ru" presents an overview of modern methods of photographing the microworld.
Strictly speaking, there is almost no ordinary photography left these days. Images that we habitually call photographs and can be found, for example, in any Lenta.ru photo essay, are actually computer models. A light-sensitive matrix in a special device (traditionally it is still called a “camera”) determines the spatial distribution of light intensity in several different spectral ranges, the control electronics stores this data in digital form, and then another electronic circuit, based on this data, gives a command to the transistors in the liquid crystal display . Film, paper, special solutions for their processing - all this has become exotic. And if we remember the literal meaning of the word, then photography is “light painting”. So what to say that the scientists succeeded to photograph an atom, is possible only with a fair amount of conventionality.
More than half of all astronomical images have long been taken by infrared, ultraviolet and X-ray telescopes. Electron microscopes irradiate not with light, but with an electron beam, while atomic force microscopes scan the relief of the sample with a needle. There are X-ray microscopes and magnetic resonance imaging scanners. All these devices give us accurate images of various objects, and despite the fact that it is, of course, not necessary to speak of "light painting" here, we still allow ourselves to call such images photographs.
Experiments by physicists to determine the shape of a proton or the distribution of quarks inside particles will remain behind the scenes; our story will be limited to the scale of atoms.
Optics never get old
As it turned out in the second half of the 20th century, optical microscopes still have room to develop. A decisive moment in biological and medical research was the emergence of fluorescent dyes and methods that allow selective labeling of certain substances. It wasn't "just new paint", it was a real revolution.
Contrary to common misconception, fluorescence is not a glow in the dark at all (the latter is called luminescence). This is the phenomenon of the absorption of quanta of a certain energy (say, blue light) with the subsequent emission of other quanta of lower energy and, accordingly, a different light (when blue is absorbed, green will be emitted). If you put in a filter that allows only the quanta emitted by the dye to pass through and blocks the light that causes fluorescence, you can see a dark background with bright spots of dyes, and dyes, in turn, can color the sample extremely selectively.
For example, you can color the cytoskeleton of a nerve cell red, highlight the synapses in green, and highlight the nucleus in blue. You can make a fluorescent label that will allow you to detect protein receptors on the membrane or molecules synthesized by the cell under certain conditions. The method of immunohistochemical staining has revolutionized biological science. And when genetic engineers learned how to make transgenic animals with fluorescent proteins, this method experienced a rebirth: mice with neurons painted in different colors became a reality, for example.
In addition, engineers came up with (and practiced) a method of so-called confocal microscopy. Its essence lies in the fact that the microscope focuses on a very thin layer, and a special diaphragm cuts off the light created by objects outside this layer. Such a microscope can sequentially scan a sample from top to bottom and obtain a stack of images, which is a ready-made basis for a three-dimensional model.
The use of lasers and sophisticated optical beam control systems has made it possible to solve the problem of dye fading and drying of delicate biological samples under bright light: the laser beam scans the sample only when it is necessary for imaging. And in order not to waste time and effort on examining a large preparation through an eyepiece with a narrow field of view, the engineers proposed an automatic scanning system: you can put a glass with a sample on the object stage of a modern microscope, and the device will independently capture a large-scale panorama of the entire sample. At the same time, in the right places, he will focus, and then glue many frames together.
Some microscopes can accommodate live mice, rats, or at least small invertebrates. Others give a slight increase, but are combined with an X-ray machine. Many are mounted on special tables weighing several tons indoors with a carefully controlled microclimate to eliminate vibration interference. The cost of such systems exceeds the cost of other electron microscopes, and competitions for the most beautiful frame have long become a tradition. In addition, the improvement of optics continues: from the search for the best types of glass and the selection of optimal lens combinations, engineers have moved on to ways to focus light.
We have specifically listed a number of technical details in order to show that progress in biological research has long been associated with progress in other areas. If there were no computers capable of automatically counting the number of stained cells in several hundred photographs, supermicroscopes would be of little use. And without fluorescent dyes, all millions of cells would be indistinguishable from each other, so it would be almost impossible to follow the formation of new ones or the death of old ones.

In fact, the first microscope was a clamp with a spherical lens attached to it. An analogue of such a microscope can be a simple playing card with a hole made in it and a drop of water. According to some reports, such devices were used by gold miners in Kolyma already in the last century.
Beyond the diffraction limit
Optical microscopes have a fundamental drawback. The fact is that it is impossible to restore the shape of those objects that turned out to be much smaller than the wavelength from the shape of light waves: you can just as well try to examine the fine texture of the material with your hand in a thick welding glove.
The limitations created by diffraction have been partly overcome, and without violating the laws of physics. Two circumstances help optical microscopes dive under the diffraction barrier: the fact that during fluorescence quanta are emitted by individual dye molecules (which can be quite far apart from each other), and the fact that by superimposing light waves it is possible to obtain a bright spot with a diameter smaller than wavelength.
When superimposed on each other, light waves are able to cancel each other out, therefore, the illumination parameters of the sample are such that the smallest possible area falls into the bright region. In combination with mathematical algorithms that can, for example, remove ghosting, such directional lighting provides a dramatic improvement in image quality. It becomes possible, for example, to examine intracellular structures with an optical microscope and even (by combining the described method with confocal microscopy) to obtain their three-dimensional images.
Electron microscope before electronic instruments
In order to discover atoms and molecules, scientists did not have to look at them - molecular theory did not need to see the object. But microbiology became possible only after the invention of the microscope. Therefore, at first, microscopes were associated precisely with medicine and biology: physicists and chemists who studied much smaller objects managed by other means. When they also wanted to look at the microcosm, diffraction limitations became a serious problem, especially since the methods of fluorescence microscopy described above were still unknown. And there is little sense in increasing the resolution from 500 to 100 nanometers if the object to be considered is even less!
Knowing that electrons can behave both as a wave and as a particle, physicists from Germany created an electron lens in 1926. The idea underlying it was very simple and understandable to any schoolchild: since the electromagnetic field deflects electrons, it can be used to change the shape of the beam of these particles by pulling them apart, or, on the contrary, to reduce the diameter of the beam. Five years later, in 1931, Ernst Ruska and Max Knoll built the world's first electron microscope. In the device, the sample was first illuminated by an electron beam, and then the electron lens expanded the beam that passed through before it fell on a special luminescent screen. The first microscope only gave a magnification of 400 times, but the replacement of light with electrons paved the way for photographing with magnification hundreds of thousands of times: the designers had only to overcome a few technical obstacles.

The electron microscope made it possible to examine the structure of cells in a quality that was previously unattainable. But from this picture it is impossible to understand the age of the cells and the presence of certain proteins in them, and this information is very necessary for scientists.
Electron microscopes now allow close-up photographs of viruses. There are various modifications of devices that allow not only to shine through thin sections, but also to consider them in "reflected light" (in reflected electrons, of course). We will not talk in detail about all the options for microscopes, but we note that recently researchers have learned how to restore an image from a diffraction pattern.
Touch, not see
Another revolution came at the expense of a further departure from the principle of "illuminate and see." An atomic force microscope, as well as a scanning tunneling microscope, no longer shines on the surface of the samples. Instead, a particularly thin needle moves across the surface, which literally bounces even on bumps the size of a single atom.
Without going into the details of all such methods, we note the main thing: the needle of a tunneling microscope can not only be moved along the surface, but also used to rearrange atoms from place to place. This is how scientists create inscriptions, drawings and even cartoons in which a drawn boy plays with an atom. A real xenon atom dragged by the tip of a scanning tunneling microscope.
The tunneling microscope is called because it uses the effect of tunneling current flowing through the needle: electrons pass through the gap between the needle and the surface due to the tunneling effect predicted by quantum mechanics. This device requires a vacuum to operate.
The atomic force microscope (AFM) is much less demanding on environmental conditions - it can (with a number of limitations) work without air pumping. In a sense, the AFM is the nanotech successor to the gramophone. A needle mounted on a thin and flexible cantilever bracket ( cantilever and there is a “bracket”), moves along the surface without applying voltage to it and follows the relief of the sample in the same way as the gramophone needle follows along the grooves of a gramophone record. The bending of the cantilever causes the mirror fixed on it to deviate, the mirror deflects the laser beam, and this makes it possible to very accurately determine the shape of the sample under study. The main thing is to have a fairly accurate system for moving the needle, as well as a supply of needles that must be perfectly sharp. The radius of curvature at the tips of such needles may not exceed one nanometer.
AFM allows you to see individual atoms and molecules, but, like a tunneling microscope, it does not allow you to look under the surface of the sample. In other words, scientists have to choose between being able to see atoms and being able to study the entire object. However, even for optical microscopes, the insides of the studied samples are not always accessible, because minerals or metals usually transmit light poorly. In addition, there are still difficulties with photographing atoms - these objects appear as simple balls, the shape of electron clouds is not visible in such images.
Synchrotron radiation, which occurs during the deceleration of charged particles dispersed by accelerators, makes it possible to study the petrified remains of prehistoric animals. By rotating the sample under X-rays, we can get three-dimensional tomograms - this is how, for example, the brain was found inside the skull of fish that became extinct 300 million years ago. You can do without rotation if the registration of the transmitted radiation is by fixing the x-rays scattered due to diffraction.
And this is not all the possibilities that X-rays open up. When irradiated with it, many materials fluoresce, and the chemical composition of a substance can be determined by the nature of the fluorescence: in this way, scientists color ancient artifacts, the works of Archimedes erased in the Middle Ages, or the color of feathers of long-extinct birds.
Posing atoms
Against the backdrop of all the possibilities provided by X-ray or optical fluorescence methods, a new way of photographing individual atoms no longer seems like such a big breakthrough in science. The essence of the method that made it possible to obtain the images presented this week is as follows: electrons are plucked from ionized atoms and sent to a special detector. Each act of ionization strips an electron from a certain position and gives one point on the "photo". Having accumulated several thousand such points, scientists formed a picture showing the most likely places for finding an electron around the nucleus of an atom, and this, by definition, is an electron cloud.
In conclusion, let's say that the ability to see individual atoms with their electron clouds is more like a cherry on the cake of modern microscopy. It was important for scientists to study the structure of materials, to study cells and crystals, and the development of technologies resulting from this made it possible to reach the hydrogen atom. Anything less is already the sphere of interest of specialists in elementary particle physics. And biologists, materials scientists and geologists still have room to improve microscopes even with a rather modest magnification compared to atoms. Experts in neurophysiology, for example, have long wanted to have a device that can see individual cells inside a living brain, and the creators of rovers would sell their souls for an electron microscope that would fit on board a spacecraft and could work on Mars.
Physicists from the United States managed to capture individual atoms in a photo with a record resolution, Day.Az reports with reference to Vesti.ru
Scientists from Cornell University in the United States managed to capture individual atoms in a photo with a record resolution of less than half an angstrom (0.39 Å). Previous photographs had half the resolution - 0.98 Å.
Powerful electron microscopes that can see atoms have been around for half a century, but their resolution is limited by the long wavelength of visible light, which is larger than the diameter of an average atom.
Therefore, scientists use a kind of analogue of lenses that focus and magnify the image in electron microscopes - they are a magnetic field. However, fluctuations in the magnetic field distort the result. To remove distortions, additional devices are used that correct the magnetic field, but at the same time increase the complexity of the electron microscope design.
Previously, physicists at Cornell University developed the Electron Microscope Pixel Array Detector (EMPAD), which replaces a complex system of generators that focus incoming electrons with a single small 128x128 pixel array that is sensitive to individual electrons. Each pixel registers the angle of electron reflection; Knowing it, scientists using the technique of ptyicography reconstruct the characteristics of the electrons, including the coordinates of the point from which it was released.

Atoms in the highest resolution
David A. Muller et al. Nature, 2018.
In the summer of 2018, physicists decided to improve the quality of the resulting images to a record-breaking resolution to date. Scientists fixed a sheet of 2D material - molybdenum sulfide MoS2 - on a movable beam, and released electron beams by turning the beam at different angles to the electron source. Using EMPAD and ptyicography, the scientists determined the distances between individual molybdenum atoms and obtained an image with a record resolution of 0.39 Å.
"In fact, we have created the smallest ruler in the world," explains Sol Gruner (Sol Gruner), one of the authors of the experiment. In the resulting image, it was possible to see sulfur atoms with a record resolution of 0.39 Å. Moreover, we even managed to see the place where one such atom is missing (indicated by an arrow).

Sulfur atoms at record resolution

The scourge of the late 20th century that caused the death of Freddy Mercury, annually carrying thousands of people beyond the line of no return to the world of the living.
The enemy of humanity must be known in, we look and remember the molecule of the AIDS Virus, which in scientific circles goes under the pseudonym HIV.

This is approximately the way cells divide into their own kind.
In the picture, the moment of division of the yeast cell.

Any biological being, whether a person or a plant, is made up of genes.
A whole chain of genes, in principle, on which much depends, due to the lack of certain genes, a person easily turns into a plant. The reverse process has not yet been observed in nature.
In the picture, the plant gene is Arabidopsis, here it is in 3D.

Yes, probably any student will recognize this picture!
A tomato seed surrounded by tiny hairs that feel like slime to the touch. Protecting the seed from premature drying.

Here it is, the longed-for dream of the majority of mankind!
For the possession of this, long and bloody wars were fought, passers-by were killed and robbed in the gateway. The whole history of mankind is involved in this.