Today there will be an industrial report from the Beauty and the Beast series.
When you take out a bright ball from a beautiful package, which you then hang on a New Year tree, you probably don’t even think about where and how it was made.
Yes, the bulk of Christmas decorations now come from China, but still not all.
There are four enterprises in Ukraine that produce good old glass Christmas decorations and it is quite possible that some of the balls on your Christmas tree are made on one of them.
For example, at a completely old-school factory, which is located not far from Kyiv.
It's hard to believe that colorful balloons can be produced in such an entourage trashy place.
Also, how hard it is to believe that each ball is blown by workers to the detriment of their health, because the production of Christmas decorations is incredibly harmful.
So, welcome to the place where the real soviet trash and the magic of the holiday, loved by millions of people, are wonderfully combined - the Klavdiev Christmas tree decorations factory.
2. The Klavdievskaya factory has been operating since the distant 1949 and today is gradually getting out of the long-term crisis caused by the collapse of the USSR.
I can imagine how difficult it is for an enterprise that operates only 2 months a year to survive in the face of the massive expansion of cheap Chinese consumer goods.
This is probably why I don’t want to paint this report in critical colors, but just talk about how ordinary people in an old Soviet factory make beauty for the main holiday of the year 
3. The factory has four workshops, which are located in different buildings.
It all starts with the glass blower, which is located in this old two-story building with cheerful multi-colored pipes. 
4. The factory has found a great way to increase profits in addition to the main activity - the production of toys.
It allows you to pay money to see how it's done.
And this is a huge respect! I love doing industrial reporting and it usually takes some effort to get a production shot.
And here the enterprise itself shows how everything is done.
And, it should be noted, very successfully.
We shot on a weekday and the traffic was incredible. Literally crowds of children and all arriving and arriving buses. 
5. Scoop here, of course, everywhere.
And it's very ambivalent.
This photo shows two completely different generations of slogans) 
6. I wonder what party they work for here now) 
7. Glassblowing shop. The workplace is simple - a gas burner, pipes with gas and compressed air, an exhaust chute and a table. 
8. Hellish labor. In the truest sense of the word. 
9. The raw material for the Christmas toy is ... hollow glass tubes.
First, they are heated and divided into blanks for balls.
Heated glass begins to melt, becomes plastic and malleable. This separates the desired parts of the tube. 
10. Then this elongated fused piece of glass is twisted with a special hook on one side to drown out this strange tube on one side.
Surprisingly, these fused pieces of glass still remain hollow inside.
After all, the ball will then be blown out. 
11. Then the blanks are again heated on the burner to the desired temperature 
12. In the hand of the future Christmas ball 
13. And when the glass reaches the right temperature, the worker simply blows into the tube, blowing out the ball. 
14. Glass should be hot, red. Its temperature is about 1000 degrees. The worker has only a few seconds to blow the balloon.
And it must be the right size.
Sometimes they check the caliber with a special measuring device, which stands on the table nearby. 
15. Ball blanks look like a large transparent drop. The leg through which the ball was blown does not break off. It will still be needed for silvering and coloring.
One worker blows 150-200 balloons per shift. 
16. Before sending to the silver plating shop 
17. This is a different building. There are workshops for silvering, coloring and decorating balls 
18. And again, a real old school - safety posters. These photos are for olgalit
. She knows everything about safety and even more. Urgently everyone friend her!!! 
19. She is spying on you! 
20. Instructions from 1989! 
21. But it's time to go to the shop. It is here, in this picturesque place, that real magic is created with balls - they are silvered 
22. Feeling as if we are in some kind of torture chamber. 
23. And here is the tool that I was talking about yesterday, offering to make assumptions about its purpose.
This is a needle for injecting a special solution into the ball, which will cover the inside of the glass ball with a thin layer of silver. pure silver. 
24. The solution consists of silver oxide, ammonia, glucose and distilled water.
It is injected quite a bit, then the workpiece is shaken so that the solution covers the walls inside the ball, and is lowered for a few seconds into a bath with water heated to 50 degrees, while shaking.
The silver solution solidifies on the walls of the ball, covering them with the thinnest uniform layer. The process is called the "silver mirror reaction".
Then the balls get to the external painting area, where they are covered with one color or another. And then they get to the most interesting part... 
25. You can’t shoot here, because it is very distracting for workers and prevents them from doing magic.
But we were kindly allowed 
25. Here the balls are turned into those beautiful Christmas decorations that we are used to.
They are painted here. 
26. Each Christmas decoration is painted manually according to a sample template. 
27. All the guys who work in this room are graduates of art schools and schools. After all, their task is to make toys amazing 
28. They draw in parts. For example, first white snow is applied to the entire batch, then a green Christmas tree, then a house, then a window in the house, etc. 
29. Ready-made toys to dry 
30. Not only balls are made here, but also various three-dimensional toys. For example, such Fushi-Mice. They are also blown, but inside a special form. 
31. Decorating a train. As for me, the balls are more beautiful and more elegant 
32. After the paint is applied, it is fixed by sprinkling with various materials. This creates the effect of rough snow or voluminous sparkles. 
33. Can you imagine the painstaking work? 
34. Stand with layout templates 
35. Workplace of the artist 
36. Half finished balls 
37. It's fun to look for various unusual labels among jars and bottles) 
38. Paint toys with acrylics 
39. Cutting and packaging area. Here the balls are circumcised - they cut off the extra leg 
40. Well, then the toys go to the store.
In the company store at the factory full house 
41. By the way, children can take a small master class where they will be taught to paint toys.
But it's not easy to get on it - there is a huge queue 
42. It's not for you to draw tanks on a notebook sheet) 
43. Add snow and - voila! 
44. Final photo. How not to cut your own bow. 
Thanks for the trip Sasha
Test " CARBOHYDRATES"
1 option
1. Carbohydrates include substances with the general formula
1) C x H y O z 2) C n (H 2 O) m 3) C n H 2n O 2 4) C n H 2n+2 O
2. Monosaccharides containing five carbon atoms are called
3. The most common hexose monosaccharide
1) glucose 2) fructose 3) ribose 4) sucrose
4. With the complete hydrolysis of polysaccharides, most often formed
1) fructose 2) glucose 3) ribose 4) galactose
5. The main function of glucose in animal and human cells
1) supply of nutrients 3) transmission of hereditary information
2) building material 4) energy source
6.
the name "grape sugar" is
7. According to its chemical structure, glucose is
8. With an ammonia solution of silver oxide, glucose reacts in the form
1) α -cyclic form 3)β -cyclic form
2) linear (aldehyde) form 4) mixturesα- and β -cyclic forms
9. A bright blue solution is formed when glucose reacts with
1) Ag 2 O / NH 3 2) Cu (OH) 2 3) H 2 / Ni 4) CH 3 COOH
10. Alcoholic fermentation of glucose produces
11. White amorphous powder, insoluble in cold water, in hot forms
colloidal solution (paste) is
12. In plant cells, starch performs the function
13. The content of amylopectin in starch is
1) 10-20% 2) 30-40% 3) 50-60% 4) 80-90%
14. The end product of starch hydrolysis is
1) maltose 2) fructose 3) glucose 4) galactose
15. With complete oxidation, 1 mol of starch is released C ABOUT 2 in quantity
1) 6 mol 2) 6 n mol 3) 12 mol 4) 12 n mol
16. The general formula of cellulose, with the release of free OH groups
1) [C 6 H 7 O 2 (OH) 3] n 2) [C 6 H 8 O 3 (OH) 2] n 3) [C 6 H 9 O 4 (OH)] n 4) [C 6 H 6 O(OH) 4 ] n
17. To distinguish glucose from fructose, use
1) H 2 /Ni 2) Ag 2 O/NH 3 3) C 2 H 5 OH/H + 4) CH 3 COOH
18. The product of glucose reduction with hydrogen on a nickel catalyst
is an
19. Determine substance B in the following transformation scheme:
Glucose A B C
1) sodium acetate 2) ethanal 3) ethyl acetate 4) ethylene
20. During lactic acid fermentation, 160 g of glucose received lactic acid with
with a yield of 85%, Determine the mass of lactic acid obtained
1) 116 g 2) 126 g 3) 136 g 4) 146 g
Test " CARBOHYDRATES"
Option 2
1. The carbohydrate is a substance
1) CH 2 O 2) C 2 H 4 O 2 3) C 5 H 10 O 5 4) C 6 H 6 O
2. Monosaccharides containing six carbon atoms are called
1) hexoses 2) pentoses 3) tetroses 4) trioses
3. To disaccharides not applicable
4. Does not apply to polysaccharides
1) starch 2) glycogen 3) cellulose 4) sucrose
5. RNA and DNA containing ribose and deoxyribose residues perform the function
6. Colorless crystalline substance, highly soluble in water,
the name "fruit sugar" is
1) sucrose 2) glucose 3) fructose 4) starch
7. Glucose isomer - fructose - is
1) acid 2) ester 3) aldehyde alcohol 4) keto alcohol
8. The product of glucose reduction with hydrogen on a nickel catalyst
is an
1) gluconic acid 2) sorbitol 3) lactic acid 4) fructose
9. The maximum number of molecules of acetic acid with which it can react
glucose in the formation of an ester, equal to
1) one 2) two 3) three 4) five
10. During lactic acid fermentation, glucose is formed
1) CH 3 COOH 2) C 2 H 5 OH 3) CH 3 CHOHCOOH 4) CH 3 CH 2 CH 2 COOH
11. Solid fibrous substance, insoluble in water
1) cellulose 2) sucrose 3) starch 4) maltose
12. In plant cells, cellulose performs the function
1) transfer of hereditary information 3) construction and structural
2) a supply of nutrients 4) a catalyst for biological processes
13. Dissolves in hot water
1) amylose 2) amylopectin 3) starch 4) cellulose
14. The general formula of cellulose, with the release of free Oh -groups
1) [ C 6 H 7 O 2 (OH) 3 ] n 2) [ C 6 H 8 O 3 (OH) 2 ] n 3) [ C 6 H 9 O 4 (OH)] n 4) [ C 6 H 6 O(OH) 4 ] n
15. The explosive substance "pyroxylin" is
1) trinitrocellulose 2) di- and triacetylcellulose
3) mononitrocellulose 4) triacetyl starch
16. General formula of polysaccharides formed by glucose
1) (CH 2 O) n 2) (C 2 H 4 O 2) n 3) (C 6 H 10 O 5) n 4) (C 6 H 6 O) n
17. Milk sugar is a disaccharide
1) sucrose 2) maltose 3) lactose 4) galactose
18. The product of glucose oxidation with an ammonia solution of silver oxide is
1) gluconic acid 2) sorbitol 3) lactic acid 4) fructose
cellulose A B C
1) glucose 2) butadiene-1,3 3) ethylene 4) ethanol
20. When 126 g of glucose interacts with an excess of ammonia solution of oxide
silver, a metal precipitate weighing 113.4 g was obtained. Determine the yield of products
percentage reactions.
1) 80 2) 75 3) 70 4) 60
Test " CARBOHYDRATES"
3 option
According to the ability of carbohydrates to hydrolyze, it is not distinguished lie group
1) monosaccharides 2) disaccharides 3) trisaccharides 4) polysaccharides
2. Pentose, which is part of RNA, is called
3. Dietary sugar is a disaccharide
1) sucrose 2) maltose 3) lactose 4) galactose
4. The general formula of polysaccharides formed by glucose
1) (CH 2 O) n 2) (C 6 H 12 O 6) n 3) (C 6 H 10 O 5) n 4) (C 6 H 6 O) n
5. For plant cells, cellulose performs the function
1) supply of nutrients 3) transmission of hereditary information
2) building material 4) energy source
6. The end products of glucose oxidation in the human body are
1) CO 2 and H 2 O 2) CO 2 and H 2 3) CO 2 and H 2 O 2 4) CO and H 2 O
7. In solution, glucose exists in the form
1) one cyclicα -forms 3) two linear forms
2) two cyclic and one linear form 4) one linear form
8. The product of glucose oxidation with an ammonia solution of silver oxide is
1) gluconic acid 2) sorbitol 3) lactic acid 4) fructose
9. The formation of a bright blue solution as a result of the interaction of glucose with C u (IS HE) 2
is proof of the presence of glucose in the molecule
1) aldehyde group 3) keto group
2) two or more hydroxo groups 4) one hydroxo group
10. In diabetes, it is used as a sugar substitute.
11. The largest amount of starch (up to 80%) is contained
1) potatoes 2) wheat 3) rice 4) corn
12. Shorter starch macromolecules with a linear structure,
called
13. Starch is a macromolecule, the structural unit of which is residues
1) αβ -cyclic form of glucose
14. In each structural unit of the cellulose molecule, the number of free
hydroxo group is equal to:
1) 1 2) 2 3) 3 4) 4
15. During the synthesis of 0.5 mol of starch in the leaves of plants, oxygen is released into
quantity
1) 6 mol 2) 6 n mol 3) 3 mol 4) 3 n mol
16. Substance belongs to carbohydrates
1) CH 2 O 2) C 2 H 4 O 2 3) C 5 H 10 O 5 4) C 6 H 6 O
17. To distinguish starch from cellulose use
1) Ag 2 O / NH 3 2) I 2 solution 3) C u (OH) 2 4) HN0 3
18. Products of the interaction of glucose with copper hydroxide ( II ) when heated
are
1) sorbitol and Cu 2 O 3) lactic acid and Cu 2 O
2) gluconic acid and Cu 2 O 4) fructose and C u
19. Determine substance B in the following transformation scheme:
starch A B C
1) glucose 2) ethanol 3) ethanal 4) acetic acid
20. Glucose was oxidized with an ammonia solution of silver oxide, thus obtaining 32.4 g
draft. Determine the mass of hexahydric alcohol that can be obtained from the same
amount of glucose, if the yield of reaction products is quantitative.
1) 27.3 g 2) 29.3 g 3) 31.3 g 4) 33.3 g
Test " CARBOHYDRATES"
4 option
Carbohydrates that are not hydrolyzed are called
1) monosaccharides 2) disaccharides 3) trisaccharides 4) polysaccharides
2. Pentose, which is part of DNA, is called
1) glucose 2) fructose 3) ribose 4) deoxyribose
3. Malt sugar is a disaccharide
1) sucrose 2) maltose 3) lactose 4) galactose
4. Sweet taste is used as a benchmark for sweetness
1) fructose 2) glucose 3) sucrose 4) galactose
5. Starch, glycogen and sucrose perform the function
1) supply of nutrients 3) transmission of hereditary information
2) building material 4) energy source
6. The energy requirement of living organisms is largely
provided by oxidation
1) sucrose 2) glucose 3) fructose 4) ribose
7. Of the three forms of existence of glucose in solution, the maximum content (about
67%) falls on
1) β -cyclic form 3) linear (aldehyde) form
2) a -cyclic form 4) mixture of linear andα -cyclic forms
8. The products of the interaction of glucose with copper hydroxide ( II ) when heated
are
1) sorbitol and C u 2 O 3) lactic acid and C u 2 O
2) gluconic acid and Cu 2 O 4) fructose and C u
9. To distinguish glucose from fructose, use
1) H 2 / Ni 2) Ag 2 O / NH 3 3) C 2 H 5 OH / H + 4) CH 3 COOH
10. In the manufacture of mirrors and Christmas decorations, it is used
1) fructose 2) starch 3) glucose 4) sorbitol
11. The largest amount of cellulose (up to 95%) is found in fibers
1) wood 2) cotton 3) linen 4) hemp
12. A part of starch with a dissolved molecular structure is called
13. Cellulose is a macromolecule, the structural unit of which is residues
1) α -cyclic form of glucose 3)β -cyclic form of glucose
2) linear form of glucose 4) linear form of fructose
14. When an ester is formed with a cellulose molecule, the maximum
react
1) Z n C 2 H 5 OH 2) 3 n CH 3 COOH 3) 2 n C 2 H 5 OH 4) 2 n CH 3 COOH
15. Rayon is a recycled product
1) trinitrocellulose 3) mononitrocellulose
2) di- and triacetylcellulose 4) triacetyl starch
16. Carbohydrates include substances with the general formula
1) B C
1) sorbitol 2) ethanol 3) ethanal 4) acetic acid
20. The mass fraction of cellulose in wood is 50%. What mass of alcohol
be obtained by hydrolysis of 100 kg of sawdust and fermentation of the resulting glucose,
if the yield of ethanol during fermentation is 75%?
1) 15.3 kg 2) 17.3 kg 3) 19.3 kg 4) 21.3 kg
Answers
1 option
1) 2;
2) 2;
3) 1;
4) 2;
5) 4;
6) 2;
7) 3;
8) 2;
9) 2;
10) 2;
11) 3;
12) 2;
13) 4;
14) 3;
15) 2;
16) 1;
17) 2;
18) 2;
19) 1;
20) 3;
Option 2
1) 3;
2) 1;
3) 4;
4) 4;
5) 3;
6) 3;
7) 4;
8) 2;
9) 4;
10) 3;
11) 1;
12) 3;
13) 1;
14) 1;
15) 1;
16) 3;
17) 3;
18) 1;
19) 2;
20) 2;
3 option
1) 3;
2) 3;
3) 1;
4) 3;
5) 2;
6) 1;
7) 2;
8) 1;
9) 2;
10) 4;
11) 3;
12) 2;
13) 1;
14) 3;
15) 4;
16) 3;
17) 2;
18) 2;
19) 3;
20) 1;
4 option
1) 1;
2) 4;
3) 2;
4) 2;
5) 1;
6) 2;
7) 1;
8) 2;
9) 2;
10) 3;
11) 2;
12) 3;
13) 3;
14) 2;
15) 2;
16) 2;
17) 1;
18) 2;
The New Year is one of the most beloved holidays, and its anticipation is sometimes more exciting than the holiday itself. One of the most enjoyable chores on the eve of the New Year is decorating the Christmas tree. And so that the Christmas tree is not dressed up with ordinary balls from the store, make Christmas tree decorations with your own hands, using school knowledge of chemistry.
From the course of organic chemistry, you are well aware of the “silver mirror” reaction, which is a qualitative reaction for an aldehyde group (the Tollens reaction). Glucose, which is an aldehyde alcohol, can be used as a reducing agent in this reaction; contains an aldehyde group. This reaction is widely used in industry for the purpose of silvering mirrors, for the manufacture of Christmas decorations, flasks for thermoses.
In an aqueous solution of ammonia, silver oxide dissolves to form a complex compound of diammine silver (I) hydroxide:
Ag 2 O + 4NH 3 H 2 O ↔ 2OH + 3H 2 O
The aldehyde group of glucose is oxidized to a carboxyl group. In this case, glucose is oxidized to gluconic acid with the formation of its ammonium salt:
CH 2 OH–(CHOH) 4 –SON + 2OH → 2Ag↓ + CH 2 OH–(CHOH) 4 –COONH 4 + 3NH 3 + H 2 O
Reagents for this reaction can be purchased at any pharmacy: lapis pencil (silver nitrate), ammonia, glucose. With this reaction, you can silver any transparent colorless or colored bottles (medicine bottles, perfume bottles, etc.)
One of the most important conditions for this reaction is the ideal cleanliness of the inner surface of the future Christmas tree toy. The main pollutant is fatty deposits. For this purpose, the inner surface is washed with an alkaline solution, after that - with repeatedly distilled (rain) water. As a last resort, you can use synthetic dishwashing detergents.
To obtain mirror coatings, it is recommended to add ammonia to silver nitrate first, and then alkali. Many methods emphasize that an excess of alkali should be avoided. Indeed, a large excess of alkali is not desirable, but do not forget that the precipitation of silver must take place in an alkaline solution.
Typically, two freshly prepared solutions are used for silvering glass, an approximate recipe for which is given below. All solutions are prepared with distilled or, in extreme cases, rain water.
Solution 1. Aqueous ammonia is added to a solution containing 6 g of AgNO3 in 100 ml of water until the initially formed precipitate dissolves, then 70 ml of a 3% NaOH solution and again aqueous ammonia until the solution is completely clarified (without excess) and the entire resulting solution is diluted with water to 500 ml.
Solution 2. A solution containing 1.3 g of glucose in 25 ml of water is boiled for 2 minutes, cooled and diluted with an equal volume of alcohol. Before use, solutions 1 and 2 are mixed in a ratio of 10:1. The silvery color appears after about 30 minutes. If necessary, to obtain a thicker layer of silver, the treatment is repeated with fresh portions of the solutions one or two more times. The resulting silver coating is washed with water.
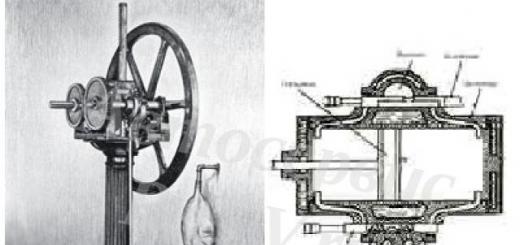
Eyelets for hanging toys on a Christmas tree are usually made of steel wire or a match inserted inside the toy, to the middle of which a thread is tied.
2. Rudzitis G.E. Chemistry. Organic chemistry. Grade 10: textbook. for general education organizations with app. to an electron. wearpelle (DVD)/G.E. Rudzitis, F.G. Feldman. - 18th ed. - M.: Education, 2014. - 191 p.: ill.
Leading: Hello dear friends! We begin our meeting of the Club of Cheerful and Resourceful. Today we will witness battles between the Vobla and SOS teams.
Teams enter the hall to the music and applause of the fans.
Leading: A respected jury will help us resolve disputes and evaluate upcoming competitions: the school principal, head teacher, teachers of chemistry, physics, literature, and two students.
So, the first round of our meeting! The gong sounds!
Contest "Cheers"
I ask the team captains to come to the jury table for the draw.
The captains choose tickets from #1 and #2.
Team greeting "sos»
Compete with you
We are humor, and laughter, and deeds.
We welcome you, friends!
You can't live without humor and laughter!
The purpose of our meeting is a joke and communication,
An exchange of fun, a look, an opinion.
We are happy to win and lose.
Let's cross in the game like swords, looks!
Greetings from the Vobla team
How many erudite people are here,
Nowhere for an apple to fall.
It will be hard for us to win
Let's not hit the face in the dirt.
Team's message "SOS» to fans
How many good girls
Gathered around here now
But one thought worries me:
Will they support SOS, my friend?
In the game we will not rest,
And you have to be strong in spirit.
Love cheerleaders restless
Help to survive, win!
Leading: We ask the jury to sum up the results of the first round of the "Greetings" competition.
The results are announced and posted on the scoreboard.
Leading: We begin the second competition: "Decipher the name of the team." The word is given to the Vobla team.
We took "Vobla" in the literal sense,
And not in any dissent,
And we dedicate an ode to this fish,
For us, and for you, and for everyone.
Ode to Woble
In those old, distant years
You were the prom queen.
Now you've become an exhibit
You go into oblivion forever.
You were eaten often, a lot, tasty,
With potatoes, with beer, with cabbage,
Saved the common people from hunger,
And now you are almost gone.
So as not to show you in museums,
Like dinosaurs, extinct animals,
For the joy of human existence.
And wobble we all shouted "SOS",
And we really love her.
Save the Remaining - Creation
Mother Nature,
So that she does not go into legends,
She was an idol for people!
And it's good that we called
Team "Vobloy" dear,
Solemnly recognized
Live, real, not a dream.
Leading: The word is given to the "SOS" command.
"SOS" - short - save souls,
Nature has been crying out to everyone for a long time.
Close your eyes, close your ears
And to survive is not meant to be...
Let's sound the alarm together
Because everything is in the answer
How can we live in the new century?
Leading: We begin the third round of our competition "Warm-up".
Competition conditions: each team prepared three questions with their answers and asks them in turn to each other. Thinking time 30 seconds. The jury evaluates the speed, correctness and originality of the answers.
Options for questions and answers for students in grade 10
1. What substance is extinguished with water, although it does not burn? - Calcium oxide (CaO) - quicklime.
2. What acid is always found in the human stomach, and when it is deficient, is it drunk as a medicine? - Hydrochloric acid (HCL).
3. What royal drink did not a single king drink? Name its composition. - Royal vodka. It is a mixture of concentrated nitric and hydrochloric acids in a ratio of 1:3.
4. What effect does chloroform have on the human body? - According to the nature of the action, chloroform is a drug. It causes dermatitis, eczema, gastrointestinal disorders. A slight poisoning is accompanied by vomiting, pain in the stomach.
5. Why do smart housewives put a few ripe apples to them when storing potatoes in the basement? - Ripe fruits of apples emit ethylene gas, which causes inhibition of growth processes. Potato tubers last longer and do not germinate.
6. Why can't you make "houses" for birds out of plastic? - Unlike wood, plastic is not able to absorb moisture and release it to the outside, so the water vapor released during breathing accumulates, forming high humidity, which is detrimental to birds.
7. What is the source of anthropogenic methane? What effect does it have on the Earth's atmosphere? - Such sources are rice fields, livestock farms, coal mines and garbage dumps. Anthropogenic methane, absorbing infrared radiation of the earth's surface, enhances the greenhouse effect.
8. Which combustion products are emitted by coal-fired thermal power plants? How do they affect the atmosphere? - Combustion products are CO2, CO, 5O2, ash, etc., they enhance the greenhouse effect.
Questions and answersFor 11th grade students
1. What kind of flour is inedible? - Inedible flour, but giving bread, these are mineral fertilizers. This is bone, phosphorite, apathetic flour.
2. What is the relationship between beets and cake? - Both products contain sugar.
3. What kind of sugar is not eaten? - Lead sugar, i.e. lead acetate, tastes sweet, but it is poisonous.
4. What oil is called Provence and why? -The best grade of olive oil (transparent, fragrant, solidifying at -60°C, Provence oil is named after the province of Provence in southern France).
5. Why are concrete trenches being destroyed, through which waste from fat-packing plants is drained? - Waste waste from fat-combining plants always contains fatty acids, which form calcium soap with cement lime, as a result of which the cement is destroyed and the bond between concrete grains is broken.
6. What is the relationship between glucose and Christmas decorations? - Since glucose contains an aldehyde group, it gives a “silver mirror” reaction with an ammonia solution of silver oxide, as a result of the reaction a thin layer of silver is released, which is covered with Christmas decorations.
7. In 1845, the German chemist Christian. Shenbein accidentally spilled a mixture of sulfuric and nitric acid on the floor. He automatically wiped the floor with his wife's cotton apron, rinsed it, and hung it up to dry over the stove. The apron dried up, but then there was a not very loud explosion and ... the apron was gone. Why did the apron explode? - Threads of cotton fabric are made of cellulose, the composition of the elementary unit of the monomer of which includes three OH groups (hydroxo groups). Under the action of acids, a nitration reaction occurs with the formation of nitrocellulose (pyroxylin), which explodes with the release of heat and gases.
The New Year is getting closer and we are beginning to be interested in its history. Quite often, the question may come to mind - where did the Christmas decorations come from? Why do they look like this? In fact, there can be many such questions. In this article, we will go back a little in history and learn more about Christmas tree decorations.
In times when there were still Celts, it was customary to worship natural forces. As you might have guessed, they believed that the earth was inhabited by a huge number of intelligent beings. To get their favor, they had to make sacrifices. They believed that even spirits live in the branches of the apple tree. The apple tree was a sacred tree. So they dressed up the apple tree in order to appease the spirits. It is from this custom that it became fashionable in Europe to decorate a Christmas tree.
The first Christmas decorations were exclusively edible. It could be candies or other goodies that could decorate the Christmas tree and give a sense of celebration. It is important that each such decoration meant something. Yes, and they hung out randomly. The next stage in the development of Christmas toys was that they began to be covered with paint. Apples were the first to be painted. Why exactly them? Apparently all because of the same Celtic custom. Previously, they believed that by dressing up the Christmas tree, in this way, it would work like a talisman and throughout the coming year it would drive away evil spirits. We can safely say that apples have become the very first Christmas tree decoration.
Over time, master glaziers began to blow balls out of glass. Such balls originally also looked like an apple. Later they began to make free-form toys. Christmas decorations have become very popular in Germany. There, glass toys were very expensive and many people simply could not afford them. But they found a way out and started making Christmas decorations with their own hands. So it has become for many a fascinating pastime and at the same time work.
The most common type of Christmas decorations was the bell. Its appearance is also due to customs. He was a powerful guardian. A little later, the Christmas tree was also decorated with flowers. Toys symbolizing Jesus Christ have also become popular. It can be concluded that now the process of decorating has lost all its former meaning. Now it doesn’t matter where we hang which toy, and even more so, we don’t even know what it symbolizes. We do this solely for beauty and creating a festive atmosphere.
Today, toys look almost unchanged. Only the material has changed. Now they are almost all, with a few exceptions, made of plastic. On the one hand, this is even good - they have become cheaper and stronger. The assortment today is simply huge and it will not be difficult for anyone to pick up something original and different from what your friends have. Every year, the number of varieties of Christmas decorations grows significantly. Here is such a story about Christmas decorations.