Fats, polysaccharides and nucleic acids, there are several thousand other organic compounds. They can be divided into final and intermediate products of biosynthesis and decomposition.
The end products of biosynthesis are organic compounds that play an independent role in the body or serve as monomers for the synthesis of biopolymers. The final products of biosynthesis include amino acids, from which proteins are synthesized in cells; nucleotides - monomers from which nucleic acids (RNA and DNA) are synthesized; glucose, which serves as a monomer for the synthesis of glycogen, starch, and cellulose.
The path to the synthesis of each of the final products lies through a series of intermediate compounds. Many substances undergo enzymatic breakdown and breakdown in cells.
Let's look at some final organic compounds.
Adenosine phosphoric acids. A particularly important role in the bioenergetics of the cell is played by the adenyl nucleotide, to which two more phosphoric acid residues are attached. This substance is called adenosine triphosphoric acid (ATP). Energy (E) is stored in the chemical bonds between the phosphoric acid residues of the ATP molecule, which is released when the phosphate is removed:
ATP - ADP+P+E
This reaction produces adenosine diphosphoric acid (ADP) and phosphoric acid (phosphate, P).
All cells use ATP energy for the processes of biosynthesis, movement, heat production, transmission of nerve impulses, luminescence (for example, in luminescent bacteria), i.e. for all vital processes.
ATP is a universal biological energy accumulator. The light energy of the Sun and the energy contained in the food consumed are stored in ATP molecules.
Regulatory and signaling substances. The final products of biosynthesis are substances that play an important role in the regulation of physiological processes and the development of the body. These include many animal hormones. Along with the protein hormones discussed in § 4, hormones of a non-protein nature are known. Some of them regulate the content of sodium ions and water in the body of animals, others ensure puberty and play an important role in the reproduction of animals. Hormones of anxiety or stress (for example, adrenaline) under stress increase the release of glucose into the blood, which ultimately leads to an increase in ATP synthesis and the active use of energy stored by the body.
Insects produce a number of special odorous substances that act as signals indicating the presence of food, danger, and attracting females to males (and vice versa).
Plants have their own hormones. Under the influence of certain hormones, the maturation of plants is significantly accelerated and their productivity increases.
Plants produce hundreds of different volatile and nonvolatile compounds that attract pollen-bearing insects; repel or poison insects that feed on plants; sometimes suppress the development of plants of other species growing nearby and competing for minerals in the soil.
Vitamins. The final products of biosynthesis include vitamins. These include vital compounds that organisms of a given species are not able to synthesize themselves, but must receive ready-made from the outside. For example, vitamin C (ascorbic acid) is synthesized in the cells of most animals, as well as in the cells of plants and microorganisms. Cells of humans, apes, guinea pigs, and some species of bats have lost the ability to synthesize ascorbic acid. Therefore, it is a vitamin only for humans and the listed animals. Animals are not able to synthesize vitamin PP (nicotinic acid), but all plants and many bacteria synthesize it.
Most of the known vitamins in the cell become components of enzymes and participate in biochemical reactions.
The daily human need for each vitamin is several micrograms. Only vitamin C is needed in an amount of about 100 mg per day.
The lack of a number of vitamins in the human and animal body leads to disruption of enzymes and is the cause of serious diseases - vitamin deficiencies. For example, a lack of vitamin C causes a serious disease - scurvy; with a lack of vitamin D, rickets develops in children.
Lesson topic: “ATP and other organic compounds of the cell”
The purpose of the lesson: study the structure and functions of ATP, introduce other organic compounds of the cell
During the classes.
I. Organizational moment.
II. Learning new material
What types of energy do you know? (Kinetic, potential.)
You studied these types of energy in physics lessons. Biology also has its own type of energy - the energy of chemical bonds. Let's say you drank tea with sugar. The food enters the stomach, where it is liquefied and sent to the small intestine, where it is broken down: large molecules to small ones. Those. Sugar is a carbohydrate disaccharide that is broken down into glucose. It is broken down and serves as a source of energy, i.e. 50% of the energy is dissipated in the form of heat to maintain a constant temperature of the body, and 50% of the energy, which is converted into ATP energy, is stored for the needs of the cell.
So, the purpose of the lesson is to study the structure of the ATP molecule.
The structure of ATP and its role in the cell
This is an unstable structure. If you separate 1 residue of NZP04, then ATP will go into ADP:
ATP+H2O =ADP+H3PO4+E, E=40kJ
ADP-adenosine diphosphate
ADP + H2O = AMP + H3PO4 + E, E = 40 kJ
Phosphoric acid residues are connected by a symbol, this is a high-energy bond:
When it breaks, 40 kJ of energy is released. Guys, let's write down the conversion of ADP from ATP:
III. Consolidation
Discussion of issues during a frontal conversation:
How is the ATP molecule structured?
What role does ATP play in the body?
How is ATP formed?
Why are the bonds between phosphoric acid residues called macroergic?
Structure of DNA and RNA (orally) - frontal questioning.
Construction of the second strand of DNA and mRNA
1) Which nucleotide is not part of DNA?
2) The nucleotide composition of DNA is –ATT-GCH-TAT-, then what should be the nucleotide composition of i-RNA?
3) Specify the composition of the DNA nucleotide?
4) What function does mRNA perform?
5) What are the monomers of DNA and RNA?
6) Name the main differences between mRNA and DNA.
7) A strong covalent bond in a DNA molecule occurs between:...
8) Which type of RNA molecule has the longest chains?
9) What type of RNA reacts with amino acids?
10 What nucleotides make up RNA?
Answers:
1) Uracil
2) UAA-CHTs-AUA
3) Phosphoric acid residue, deoxyribose, adenine
4) Removal and transfer of information from DNA
5) Nucleotides,
6) Single-chain, contains ribose, transmits information
7) Phosphoric acid residue and sugars of neighboring nucleotides
8) I-RNA
9) T-RNA
10) Adenine, uracil, guanine, cytosine.
V. Homework
§ 6, pp. 36-37
Preview:
- Draw a diagram of the ATP molecule using the following notation:
A – nitrogenous base (in this case, adenine)
U – carbohydrate (in this case, ribose)
F – phosphoric acid residue (phosphate)
FC - phosphoric acid
Using these notations, compose possible transformations of the ATP molecule in the cell, accompanied by the release or absorption of energy
- Using the suggested scheme, name the word:
A) __ __b__ __ __
Part of the ATP molecule
B) __ __e__ __e__ __ __e__ __ __ __
Function of ATP in the cell
B) __ __ __ e__o__ __
Substances whose breakdown (splitting) is one of the conditions for the synthesis of ATP molecules
- Compare the processes of cellular respiration in mitochondria (A) and combustion processes in inanimate nature (B), highlighting similarities and differences.
- Refers to oxidation reactions
- ATP synthesis occurs
- Enzymes take part in reactions
- The end products of the reaction are carbon dioxide and water
- The reaction releases thermal energy
- Refers to dissimilation reactions
Question 1. What is the structure of the ATP molecule?
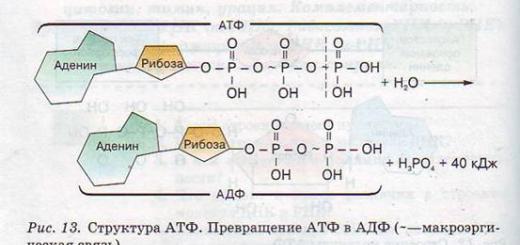
ATP is adenosine triphosphate, a nucleotide belonging to the group of nucleic acids. The concentration of ATP in the cell is low (0.04%; in skeletal muscles 0.5%). The adenosine triphosphoric acid (ATP) molecule in its structure resembles one of the nucleotides of the RNA molecule. ATP includes three components: adenine, the five-carbon sugar ribose and three phosphoric acid residues, interconnected by special high-energy bonds.
Question 2. What is the function of ATP?
ATP is a universal source of energy for all reactions occurring in the cell. Energy is released when phosphoric acid residues are separated from the ATP molecule when high-energy bonds are broken. The bond between phosphoric acid residues is high-energy; its cleavage releases approximately 4 times more energy than the cleavage of other bonds. If one phosphoric acid residue is separated, then ATP turns into ADP (adenosine diphosphoric acid). This releases 40 kJ of energy. When the second phosphoric acid residue is separated, another 40 kJ of energy is released, and ADP is converted to AMP (adenosine monophosphate). The released energy is used by the cell. The cell uses ATP energy in biosynthesis processes, during movement, during heat production, during nerve impulses, during photosynthesis, etc. ATP is a universal energy accumulator in living organisms.
During the hydrolysis of a phosphoric acid residue, energy is released:
ATP + H 2 O = ADP + H 3 PO 4 + 40 kJ/mol
Question 3. What connections are called macroergic?
The bonds between phosphoric acid residues are called macroergic, since their rupture releases a large amount of energy (four times more than the cleavage of other chemical bonds).
Question 4. What role do vitamins play in the body?
Metabolism is impossible without the participation of vitamins. Vitamins are low-molecular organic substances vital for the existence of the human body. Vitamins are either not produced at all in the human body, or are produced in insufficient quantities. Since vitamins are most often the non-protein part of enzyme molecules (coenzymes) and determine the intensity of many physiological processes in the human body, their constant intake into the body is necessary. Exceptions to some extent are vitamins B and A, which can accumulate in small quantities in the liver. In addition, some vitamins (B 1 B 2, K, E) are synthesized by bacteria living in the large intestine, from where they are absorbed into the human blood. If there is a lack of vitamins in food or diseases of the gastrointestinal tract, the supply of vitamins in the blood decreases, and diseases generally called hypovitaminosis occur. In the complete absence of any vitamin, a more severe disorder occurs, called vitamin deficiency. For example, vitamin D regulates the exchange of calcium and phosphorus in the human body, vitamin K is involved in the synthesis of prothrombin and promotes normal blood clotting.
Vitamins are divided into water-soluble (C, PP, B vitamins) and fat-soluble (A, D, E, etc.). Water-soluble vitamins are absorbed in an aqueous solution, and when they are in excess in the body, they are easily excreted in the urine. Fat-soluble vitamins are absorbed along with fats, so impaired digestion and absorption of fats is accompanied by a lack of vitamins (A, O, K). A significant increase in the content of fat-soluble vitamins in food can cause a number of metabolic disorders, since these vitamins are poorly excreted from the body. Currently, there are at least two dozen substances related to vitamins.
>> ATP and other organic compounds of the cell
ATP and other organic compounds of the cell.
1. What organic substances do you know?
2. What vitamins do you know? What is their role?
3. What types of energy do you know?
4. Why is energy necessary for the life of any organism?
Adenosine triphosphate (ATP) is a nucleotide consisting of the nitrogenous base adenine, carbohydrates ribose and three phosphoric acid residues (Fig. 12), found in the cytoplasm, mitochondria, plastids and nuclei.
ATP is an unstable structure. When one phosphoric acid residue is separated, ATP turns into adenosine diphosphate (ADP), if another phosphoric acid residue is separated (which is extremely rare), then ADP turns into adenosine monophosphate (AMP). When each phosphoric acid residue is separated, 40 kJ of energy is released.
ATP + H2O → ADP + H3PO4 + 40 kJ,
ADP + H2O →AMP + H3PO4 + 40 kJ.
The bond between phosphoric acid residues is called high-energy (it is designated by the symbol -) since its rupture releases almost four times more energy than the cleavage of other chemical bonds (Fig. 13).
ATP is a universal source of energy for all reactions occurring in the cell.
Vitamins (from Latin vita - life) are complex bioorganic compounds necessary in small quantities for normal life. organisms. Unlike other organic substances, vitamins are not used as a source of energy or building material. Organisms can synthesize some vitamins themselves (for example, bacteria are able to synthesize almost all vitamins), other vitamins enter the body with food.

Vitamins are usually designated by letters of the Latin alphabet. The modern classification of vitamins is based on their ability to dissolve in water and fat. There are fat-soluble (A, D, E and K) and water-soluble (B, C, PP, etc.) vitamins.
Vitamins play a big role in metabolism and other vital processes of the body. Both deficiency and excess of vitamins can lead to serious disturbances in many physiological functions in the body.
In addition to the organic compounds listed above (carbohydrates, lipids, squirrels, nucleic acids, vitamins) there are always many other organic substances in any cell. They are intermediate or final products of biosynthesis and breakdown.
Adenosine triphosphate (ATP). Adenosine diphosphate (ADP). Adenosine monophosphate (AMP). Macroergic connection.
Vitamins are fat-soluble and water-soluble.
1. What is the structure of the ATP molecule?
2. What function does ATP perform?
3. What connections are called macroergic?
4. What role do vitamins play in the body?
Kamensky A. A., Kriksunov E. V., Pasechnik V. V. Biology 9th grade
Submitted by readers from the website
In any cell, in addition to proteins, fats, polysaccharides and nucleic acids, there are several thousand other organic compounds. They can be divided into final and intermediate products of biosynthesis and decomposition.
The end products of biosynthesis are organic compounds that play an independent role in the body or serve as monomers for the synthesis of biopolymers. The final products of biosynthesis include amino acids, from which proteins are synthesized in cells; nucleotides - monomers from which nucleic acids (RNA and DNA) are synthesized; glucose, which serves as a monomer for the synthesis of glycogen, starch, and cellulose.
The path to the synthesis of each of the final products lies through a series of intermediate compounds. Many substances undergo enzymatic breakdown and breakdown in cells.
Let's look at some final organic compounds.
Adenosine phosphoric acids. A particularly important role in the bioenergetics of the cell is played by the adenyl nucleotide, to which two more phosphoric acid residues are attached. This substance is called adenosine triphosphoric acid (ATP). Energy (E) is stored in the chemical bonds between the phosphoric acid residues of the ATP molecule, which is released when the phosphate is removed:
ATP → ADP + P + E
This reaction produces adenosine diphosphoric acid (ADP) and phosphoric acid (phosphate, P).
All cells use ATP energy for the processes of biosynthesis, movement, heat production, transmission of nerve impulses, luminescence (for example, in luminescent bacteria), i.e. for all vital processes.
ATP is a universal biological energy accumulator. The light energy of the Sun and the energy contained in the food consumed are stored in ATP molecules.
Regulatory and signaling substances. The final products of biosynthesis are substances that play an important role in the regulation of physiological processes and the development of the body. These include many animal hormones. Along with the protein hormones discussed in § 4, hormones of a non-protein nature are known. Some of them regulate the content of sodium ions and water in the body of animals, others ensure puberty and play an important role in the reproduction of animals. Hormones of anxiety or stress (for example, adrenaline) under stress increase the release of glucose into the blood, which ultimately leads to an increase in ATP synthesis and the active use of energy stored by the body.
Insects produce a number of special odorous substances that act as signals indicating the presence of food, danger, and attracting females to males (and vice versa).
Plants have their own hormones. Under the influence of certain hormones, the maturation of plants is significantly accelerated and their productivity increases.
Plants produce hundreds of different volatile and nonvolatile compounds that attract pollen-bearing insects; repel or poison insects that feed on plants; sometimes suppress the development of plants of other species growing nearby and competing for minerals in the soil.
Vitamins. The final products of biosynthesis include vitamins. These include vital compounds that organisms of a given species are not able to synthesize themselves, but must receive ready-made from the outside. For example, vitamin C (ascorbic acid) is synthesized in the cells of most animals, as well as in the cells of plants and microorganisms. Cells of humans, apes, guinea pigs, and some species of bats have lost the ability to synthesize ascorbic acid. Therefore, it is a vitamin only for humans and the listed animals. Animals are not able to synthesize vitamin PP (nicotinic acid), but all plants and many bacteria synthesize it.
Most of the known vitamins in the cell become components of enzymes and participate in biochemical reactions.
The daily human need for each vitamin is several micrograms. Only vitamin C is needed in an amount of about 100 mg per day.
The lack of a number of vitamins in the human and animal body leads to disruption of enzymes and is the cause of serious diseases - vitamin deficiencies. For example, a lack of vitamin C causes a serious disease - scurvy; with a lack of vitamin D, rickets develops in children.
- What is the importance of ATP in a cell?
- What are the final products of biosynthesis in the cell? What is their biological significance?
- What biological role do vitamins play in the body?