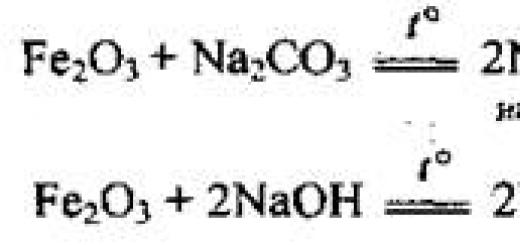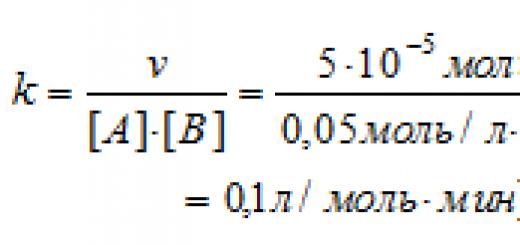This section contains all the collections of tasks in chemistry that were at the OGE 2020, 2019, 2018, 2017, 2016, 2015 of various levels of complexity. By working through these tests, you will be able to determine your level of preparation for this mandatory exam after the 9th grade.
Students completing grade 9 in the 2018-2020 academic year, like last year, will take the OGE in chemistry at their schools. The exam will be held in three stages:
- early (for those who have the right to early surrender);
- main (for the majority of graduates from all regions of the Russian Federation);
- additional (September retake).
Exam schedule (preliminary):
The structure consists of 2 blocks:
Exam without experiment
The main part of the simplified version of the OGE in chemistry will be tests. All tasks will be divided into two blocks:
Thus, in the 120 minutes (2 hours) allotted for the exam, students will have to answer 22 questions by correctly filling out the OGE form.
Exam with experiment
Graduates of specialized classes who have studied chemistry at an advanced level will not only have to give answers to test questions, but also perform a real experiment (task No. 23). In model No. 2 of 2019, tasks will be distributed:
Two hours and twenty minutes will be given to complete 23 tasks of the profile-level KIM.Can be used:
- non-programmable calculator;
- periodic table;
- electrochemical series of voltages of metals;
- table of solubility in water of salts, acids and bases;
- draft.
All entries made on the draft are not considered when evaluating, so you can safely write down everything that is necessary on the sheets and not be afraid to correct mistakes.
The scale for converting primary points into an assessment in individual regions of the Russian Federation may differ. In 2019, FIPI recommends the following compliance ranges:
Students who fail for any reason to reach the threshold of 9 points will receive 2 more attempts to retake.
Main period
Additional period (September terms)
For schoolchildren who plan to master a profession related to chemistry in the future, the OGE in this subject is very important. If you want to get the best score on the tests, start preparing immediately. The best number of points in the performance of the work is 34. The indicators of this exam can be used when sending to specialized classes in a secondary school. At the same time, the minimum limit of the indicator by points in this case is 23.
What are the options
The OGE in chemistry, as in previous years, includes theory and practice. With the help of theoretical tasks, they check how boys and girls know the basic formulas and definitions of organic and inorganic chemistry and how to apply them in practice. The second part, respectively, is aimed at testing the ability of schoolchildren to carry out reactions of the redox and ion-exchange types, to have an idea of the molar masses and volumes of substances.
Why testing is necessary
OGE 2020 in chemistry requires serious preparation, as the subject is quite complicated. Many have already forgotten the theory, perhaps they misunderstood it, and without it it is impossible to correctly solve the practical part of the task.
It is worth taking the time to train now in order to show a decent result in the future. Today, schoolchildren have an excellent opportunity to assess their strength by solving last year's real tests. No costs - you can use school knowledge for free and understand how the exam will take place. Students will be able not only to repeat the material covered and complete the practical part, but also to feel the atmosphere of real tests.
Convenient and efficient
A great opportunity is to prepare for the OGE right at the computer. You just need to press the start button and start passing the tests online. This is very effective and can replace tutoring. For convenience, all tasks are grouped by ticket numbers and fully correspond to the real ones, since they are taken from the website of the Federal Institute of Pedagogical Measurements.
If you are not confident in your abilities, you are afraid of the upcoming tests, you have gaps in theory, you have not completed enough experimental tasks, turn on the computer and start preparing. We wish you success and the highest marks!
■ Is there a guarantee that after classes with you we will pass the OGE in chemistry with the required score?
Over 80% ninth-graders who have completed a full course of preparation for the OGE and regularly completed their homework, passed this exam perfectly! And this despite the fact that even 7-8 months before the exam, many of them could not remember the formula of sulfuric acid and confused the solubility table with the periodic table!
■ Already January, knowledge of chemistry - at zero. Is it already too late or is there still a chance to pass the OGE?
There is a chance, but on condition that the student is ready to work seriously! I am not shocked by the zero level of knowledge. Moreover, most of the ninth graders are preparing for the OGE. But you need to understand that miracles do not happen. Without the active work of the student, knowledge "by itself" will not fit in the head.
■ Preparation for the OGE in chemistry - is it very hard?
First of all, it's very interesting! I cannot call the OGE in Chemistry a difficult exam: the tasks offered are quite standard, the range of topics is known, the evaluation criteria are "transparent" and logical.
■ How does the OGE exam in chemistry work?
There are two options for the OGE: with and without an experimental part. In the first version, students are offered 23 tasks, two of which are related to practical work. You have 140 minutes to complete the task. In the second option, 22 problems must be solved in 120 minutes. 19 tasks require only a short answer, the rest require a detailed solution.
■ How (technically) can I sign up for your classes?
Very simple!
- Call me on the phone: 8-903-280-81-91 . You can call any day until 23.00.
- We will arrange the first meeting for preliminary testing and determining the level of the group.
- You choose the time of classes convenient for you and the size of the group (individual lessons, classes in pairs, mini-groups).
- Everything, at the appointed time, work begins.
Good luck!
Or you can just use this site.
■ How best to prepare: in a group or individually?
Both options have their advantages and disadvantages. Classes in groups are optimal in terms of price-quality ratio. Individual lessons allow for a more flexible schedule, finer "tuning" of the course to the needs of a particular student. After preliminary testing, I will recommend you the best option, but the final choice is yours!
■ Do you make home visits to students?
Yes, I'm leaving. To any district of Moscow (including areas outside the Moscow Ring Road) and to the Moscow suburbs. At home, students can conduct not only individual, but also group classes.
■ And we live far from Moscow. What to do?
Practice remotely. Skype is our best assistant. Distance classes are no different from face-to-face: the same methodology, the same teaching materials. My login: repetitor2000. Contact us! Let's do a trial lesson - you will see how easy it is!
■ When can classes start?
Basically, at any time. The ideal option is one year before the exam. But even if there are several months left before the OGE, contact us! Perhaps there are still free "windows", and I can offer you an intensive course. Call: 8-903-280-81-91!
■ Does good preparation for the USE guarantee successful completion of the USE in chemistry in the eleventh grade?
Doesn't guarantee it, but contributes a lot to it. The foundation of chemistry is laid precisely in grades 8-9. If a student masters the basic sections of chemistry well, it will be much easier for him to study in high school and prepare for the exam. If you are planning to enter a university with a high level of requirements in chemistry (Moscow State University, leading medical universities), you should start preparing not a year before the exam, but already in grades 8-9!
■ How much will OGE-2020 in chemistry differ from OGE-2019?
No changes are planned. There are two versions of the exam: with or without a practical part. The number of tasks, their topics, and the grading system remain the same as they were in 2019.
- a scale for recalculating the primary score for completing the 2020 examination paper into a mark on a five-point scale;
- a scale for recalculating the primary score for completing the 2019 examination paper into a mark on a five-point scale;
- a scale for recalculating the primary score for the completion of the examination paper in 2018 into a mark on a five-point scale;
- a scale for recalculating the primary score for the performance of the examination paper in 2017 into a mark on a five-point scale;
- a scale for recalculating the primary score for the performance of the examination paper in 2016 into a mark on a five-point scale;
- a scale for recalculating the primary score for the performance of the examination paper in 2015 into a mark on a five-point scale;
- a scale for recalculating the primary score for the performance of the examination paper in 2014 into a mark on a five-point scale;
- a scale for recalculating the primary score for the performance of the examination paper in 2013 into a mark on a five-point scale.
Changes in the demonstration versions of the OGE in chemistry
In 2015 in demo versions of the OGE in chemistry was the structure of options has been changed:
- The variant began to consist in two parts.
- Numbering assignments has become through throughout the variant without letter designations A, B, C.
- The form of recording the answer in tasks with a choice of answers has been changed: the answer has become necessary to write digit with the number of the correct answer(not circled).
Since 2014, demonstration versions of the OGE in chemistry presented two models. These models differ only in the practice-oriented tasks of the last part, moreover, model 1 is similar to the work of previous years, and model 2 provides for the implementation real chemical experiment (tasks C3, C4 in the 2014 version and tasks 22.23 in the 2015-2016 versions). To organize and conduct a real chemical experiment in model 2, methodological materials were developed by the Federal Institute of Pedagogical Measurements. The choice of the exam model is carried out by the educational authorities of the constituent entities of the Russian Federation.
AT demo versions of the OGE 2016-2019 in chemistry compared to 2015 demos there were no changes.
In 2020, only one model of the demonstration version of the OGE in chemistry, in which, compared to the previous year 2019, the following changes:
- increased the proportion of items with multiple choice answers (6, 7, 12, 14, 15);
- increased the proportion of tasks to establish a correspondence between the positions of two sets (10, 13, 16);
- task 1 added, providing for testing the ability to work with textual information;
- in part 2 included task 21, aimed at checking the understanding of the existence of a relationship between different classes of inorganic substances and the formation of the ability to compose reaction equations that reflect this relationship. Another controlled skill is the ability to write equations for ion exchange reactions, in particular, the reduced ionic equation;
- added mandatory practical part, which includes two tasks: 23 and 24:
- in task 23 from the proposed list, it was necessary to select two substances, the interaction with which reflects the chemical properties of the substance specified in the condition of the assignment, and draw up two reaction equations with them;
- in task 24 it was necessary to carry out two reactions corresponding to the compiled reaction equations.
M.: 2017. - 320 p.
The new handbook contains all the theoretical material on the course of chemistry required to pass the main state exam in the 9th grade. It includes all elements of the content, checked by control and measuring materials, and helps to generalize and systematize knowledge and skills for the course of the secondary (complete) school. The theoretical material is presented in a concise and accessible form. Each topic is accompanied by examples of test tasks. Practical tasks correspond to the OGE format. Answers to the tests are given at the end of the manual. The manual is addressed to schoolchildren and teachers.
Format: pdf
The size: 4.2 MB
Watch, download:drive.google
CONTENT
From author 10
1.1. The structure of the atom. The structure of the electron shells of atoms of the first 20 elements of the Periodic Table D.I. Mendeleeva 12
The nucleus of an atom. Nucleons. Isotopes 12
Electronic shells 15
Electronic configurations of atoms 20
Tasks 27
1.2. Periodic law and Periodic system of chemical elements D.I. Mendeleev.
The physical meaning of the serial number of the chemical element 33
1.2.1. Groups and periods of the Periodic system 35
1.2.2. Patterns of changes in the properties of elements and their compounds in connection with the position in the Periodic system of chemical elements 37
Changing the properties of elements in the main subgroups. 37
Changing element properties by period 39
Tasks 44
1.3. The structure of molecules. Chemical bond: covalent (polar and non-polar), ionic, metallic 52
Covalent bond 52
Ionic bond 57
Metal connection 59
Tasks 60
1.4. Valency of chemical elements.
The degree of oxidation of chemical elements 63
Tasks 71
1.5. Pure substances and mixtures 74
Tasks 81
1.6. Simple and complex substances.
The main classes of inorganic substances.
Nomenclature of inorganic compounds 85
Oxides 87
Hydroxides 90
Acids 92
Salts 95
Tasks 97
2.1. Chemical reactions. Conditions and signs of chemical reactions. Chemical
equations. Conservation of the mass of substances in chemical reactions 101
Tasks 104
2.2. Classification of chemical reactions
on various grounds: the number and composition of the starting and obtained substances, changes in the oxidation states of chemical elements,
absorption and release of energy 107
Classification according to the number and composition of reagents and final substances 107
Classification of reactions according to the change in the oxidation states of chemical elements HO
Classification of reactions according to the thermal effect 111
Tasks 112
2.3. Electrolytes and non-electrolytes.
Cations and anions 116
2.4. Electrolytic dissociation of acids, alkalis and salts (medium) 116
Electrolytic dissociation of acids 119
Electrolytic dissociation of bases 119
Electrolytic dissociation of salts 120
Electrolytic dissociation of amphoteric hydroxides 121
Tasks 122
2.5. Ion exchange reactions and conditions for their implementation 125
Examples of writing reduced ionic equations 125
Conditions for the implementation of ion exchange reactions 127
Tasks 128
2.6. Redox reactions.
Oxidizing and reducing agents 133
Classification of redox reactions 134
Typical reducing and oxidizing agents 135
Selection of coefficients in the equations of redox reactions 136
Tasks 138
3.1. Chemical properties of simple substances 143
3.1.1. Chemical properties of simple substances - metals: alkali and alkaline earth metals, aluminum, iron 143
Alkali metals 143
Alkaline earth metals 145
Aluminum 147
Iron 149
Tasks 152
3.1.2. Chemical properties of simple substances - non-metals: hydrogen, oxygen, halogens, sulfur, nitrogen, phosphorus,
carbon, silicon 158
Hydrogen 158
Oxygen 160
Halogens 162
Sulfur 167
Nitrogen 169
Phosphorus 170
Carbon and silicon 172
Tasks 175
3.2. Chemical properties of complex substances 178
3.2.1. Chemical properties of oxides: basic, amphoteric, acidic 178
Basic oxides 178
Acid oxides 179
Amphoteric oxides 180
Tasks 181
3.2.2. Chemical properties of bases 187
Tasks 189
3.2.3. Chemical properties of acids 193
General properties of acids 194
Specific properties of sulfuric acid 196
Specific properties of nitric acid 197
Specific properties of phosphoric acid 198
Tasks 199
3.2.4. Chemical properties of salts (medium) 204
Tasks 209
3.3. The relationship of different classes of inorganic substances 212
Tasks 214
3.4. Initial information about organic substances 219
Main classes of organic compounds 221
Fundamentals of the theory of the structure of organic compounds ... 223
3.4.1. Limit and unsaturated hydrocarbons: methane, ethane, ethylene, acetylene 226
Methane and ethane 226
Ethylene and acetylene 229
Tasks 232
3.4.2. Oxygen-containing substances: alcohols (methanol, ethanol, glycerin), carboxylic acids (acetic and stearic) 234
Alcohols 234
Carboxylic acids 237
Tasks 239
4.1. Rules for safe work in the school laboratory 242
Rules for safe work in the school laboratory. 242
Laboratory glassware and equipment 245
Separation of mixtures and purification of substances 248
Preparation of solutions 250
Tasks 253
4.2. Determination of the nature of the environment of solutions of acids and alkalis using indicators.
Qualitative reactions to ions in solution (chloride, sulfate, carbonate ions) 257
Determination of the nature of the environment of solutions of acids and alkalis using indicators 257
Qualitative reactions to ions
in solution 262
Tasks 263
4.3. Qualitative reactions to gaseous substances (oxygen, hydrogen, carbon dioxide, ammonia).
Obtaining gaseous substances 268
Qualitative reactions to gaseous substances 273
Tasks 274
4.4. Carrying out calculations based on formulas and equations of reactions 276
4.4.1. Calculation of the mass fraction of a chemical element in a substance 276
Tasks 277
4.4.2. Calculating the mass fraction of a solute in a solution 279
Tasks 280
4.4.3. Calculation of the amount of a substance, mass or volume of a substance from the amount of a substance, mass or volume of one of the reagents
or reaction products 281
Calculating the amount of a substance 282
Mass calculation 286
Volume calculation 288
Tasks 293
Information about the two exam models of the OGE in chemistry 296
Instructions for the implementation of the experimental task 296
Samples of experimental tasks 298
Answers to tasks 301
Applications 310
Table of solubility of inorganic substances in water 310
Electronegativity of s- and p-elements 311
Electrochemical voltage series of metals 311
Some of the most important physical constants 312
Prefixes in the formation of multiple and submultiple units 312
Electronic configurations of atoms 313
The most important acid-base indicators 318
Geometric structure of inorganic particles 319