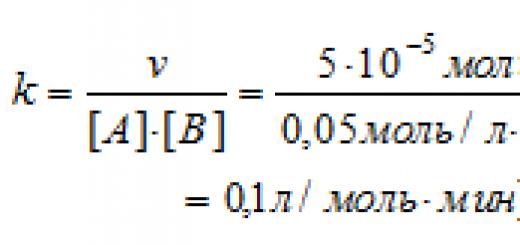Independent work in chemistry Rates of chemical reactions (catalysts) for 9th grade students. Independent work consists of 3 options in each of 4 tasks and is designed to test knowledge on the topic General characteristics of chemical elements and reactions.
1 option
1.
3 complete the reaction schemes:
a) Zn + HCl (p-p) → ...;
b) KOH (solution) + H 2 SO 4 (p-p) → ...;
c) N 2 + H 2 → ....
2.
4FeS 2 + 11O 2 = 2Fe 2 O 3 + 8SO 2
Justify your conclusions.
3.
A 10% hydrochloric acid solution was poured into test tubes with Zn, Fe, and Mg granules. In which test tube will the reaction rate be:
a) maximum;
b) minimum?
Explain the answer.
4. What can be observed in the action of hydrogen peroxide on raw and boiled meat? How to explain these phenomena?
Option 2
1.
Complete the reaction schemes:
a) CH 4 + O 2 → ...;
b) NaCl
→ … ;
c) CaCO 3 + HCl
→ … .
Indicate which of the reactions you have given are heterogeneous and which are homogeneous.
2.
Suggest ways to increase the rate of reaction
Fe + H 2 SO 4
FeSO 4 + H 2
Justify your conclusions.
3.
Three test tubes containing zinc granules were filled with 10%, 20% and 30% hydrochloric acid solution, respectively. In which test tube will the reaction rate be:
a) maximum;
b) minimum?
Explain the answer.
4. If water is added to a mixture of iodine and aluminum, then almost immediately a release of violet vapor is observed, and then a flame appears. What role does water play in this process?
3 option
1.
Complete the reaction schemes:
a) H 2 S + O 2 → ...;
b) CuS + O 2 → …;
c) FeS + HCl → … .
Indicate which of the reactions you have given are heterogeneous and which are homogeneous.
2.
Suggest ways to increase the rate of reaction
2SO 2 + O 2 \u003d 2SO 3
Justify your conclusions.
3.
Mg and Zn granules were placed in two tubes, and a 10% solution of hydrofluoric acid was poured into each tube. The same Mg and Zn granules were placed in two other test tubes and a 10% hydrochloric acid solution was added. In which test tube will the reaction rate be:
a) maximum;
b) minimum.
Explain the answer.
4. Why won't enzyme detergents help remove stains when boiled? What type of catalysts do they most likely belong to?
Answers to independent work in chemistry Rates of chemical reactions
Option 1.
1.
a) Zn + 2HCl = ZnCl 2 + H 2 heterogeneous,
b) 2KOH + H 2 SO 4 \u003d K 2 SO 4 + 2H 2 O - homogeneous,
c) N 2 + 3H 2 \u003d 2NH 3 - homogeneous.
2. Grind FeS 2, increase the concentration of O 2, its pressure, increase the temperature.
3.
a) with Mg;
b) with Fe.
The speed depends on the nature of the metal and its position in the electrochemical series of metal voltages.
4. On raw meat, the reaction is much faster, as there are enzymes - biological catalysts.
Option 2.
1.
a) CH 4 + 2O 2 \u003d CO 2 + 2H 2 O - homogeneous;
b) NaCl + AgNO 3 = AgCl + NaNO 3 - homogeneous;
c) CaCO 3 + 2HCl = CaCl 2 + CO 2 + H 2 O - heterogeneous.
2. The speed will increase if Fe is crushed, the concentration of H 2 SO 4 is increased, and the temperature is increased.
3.
a) with a 30% solution;
b) with 10% solution.
4. H 2 O - catalyst.
Option 3.
1.
a) 2H 2 S + 3O 2 \u003d 2SO 2 + 2H 2 O - homogeneous,
b) 2CuS + 3O 2 = 2CuO + 2SO 2 - heterogeneous,
c) FeS + 2HCl = FeCl 2 + H 2 S - heterogeneous.
2. Increase pressure, increase temperature, apply catalyst.
3.
a) Mg and HCl;
b) Zn and HF.
The nature of the metal and the strength of the acid are affected.
4. Enzymes are biological catalysts, they work only at temperatures up to 40 °.
Analysis of USE tasks on this topic:"The rate of a chemical reaction".
Tumanova Elena Borisovna , teacher of chemistry GBOU secondary school No. 806,
Moscow city
Etcoconducted in the late 90s. 20th century analysis of the state of the Russian education system revealed the dissatisfaction of the professional community and the public with the current practice of school final examsoin and entrance examinations in institutions of higher and secondary vocational education. The traditional final control system did not provide reliable information about the quality of general education necessary for making informed decisions in the management systemoformation.
For PoImproving the objectivity of the final certification of graduates of general educational institutions and ensuring equal opportunities for secondary and higher professional education
Wereothe goals of introducing the USE are limited:
Increasing the availability of vocational education, first of all, for capable young people from low-income families and places of residence remote from university centers;
Ensuring the continuity of general and professional education;
Ensuring nationwide control and management of the quality of education based on an independent assessment of the training of graduates.
During the years of the experiment on the introduction of the Unified State Examination, the regulatory and legal framework for conducting the exam in a new form was formed, the technology for conducting the exam, the procedure for creating control measuring materials (CMM) were worked out.
In recent years, there has been a tendency to stabilize the results of the USE. However, at the same time, typical mistakes of examinees repeating from year to year when performing tasks on the same subject are still revealed. It should be noted that tasks on the rate of chemical reactions in all the years of the USE caused certain difficulties for the majority of graduates. Students formally, without taking into account the specific conditions of the task, use knowledge about the rate of a chemical reaction and the factors influencing its change. In addition, there is a "confusion" of the concepts of "factors influencing the change in the rate of a chemical reaction" and "factors influencing the shift in chemical equilibrium." Apparently, this topic is not given enough attention in the school curriculum. In this work, I want to analyze the main mistakes of students when completing USE tasks in block A on the topic “Chemical Reaction Rate”.
Typical tasks:
Example 1
Forward reaction rate
N 2 + 3 H 2 ↔ 2 NH 3 + Q
increases with:
increase in nitrogen concentration
decrease in nitrogen concentration
increase in ammonia concentration
decrease in ammonia concentration
Recall that, in accordance with the law of mass action of Gulberg and Waage, the rate of a chemical reaction is proportional to the concentration of reactants. In this regard, answers 3 and 4 immediately fall out of consideration, requiring a change in the concentration of not the reactants, but the reaction product. A change (increase or decrease) in the concentration of ammonia will not affect the reaction rate in any way. From
the other two, we choose the first answer - it is an increase in the concentration of one of the reactants (in this case, nitrogen) that will lead to an increase in the rate of the direct reaction. Correct answer 1.
Example 2
The reaction proceeds at the highest rate under normal conditions
2 Ba+O 2 = 2BaO
Ba 2+ + CO 3 2- = BaCO 3 ↓
Ba+2H + = Ba 2+ + H 2
Ba + S = BaS
This is a problematic assignment. We often hear that schoolchildren have never seen barium and do not know how fast it reacts with oxygen or sulfur. The fact is that when studying chemistry, there is no need to get acquainted in detail with the properties of all 118 chemical elements, it is enough to study the properties of the most typical of them. And then - to use the power of the periodic law and the studied chemical theories to predict the properties of unknown substances and the laws of reactions.
Let's analyze the proposed answers. To begin with, let's compare answers 1 and 4. Barium is one of the most active metals, which is even stored under a layer of mineral oil or kerosene to prevent its oxidation by atmospheric oxygen. The reaction with oxygen proceeds quite quickly, when barium is heated in air, it effectively burns out, combining with oxygen, but in any case this is not the fastest reaction. With sulfur, barium will react much more slowly compared to oxygen.
There is no doubt that barium reacts very violently with an acid solution (answer 3). But isn't there an even faster response? It exists - it is a reaction between aqueous solutions of barium and carbonic acid. Anyone who has done this or a similar experiment in the laboratory has seen barium carbonate precipitate instantly. And this is not only a feature of barium salts. It has been established that it is the reactions in electrolyte solutions that proceed with the highest speeds - the reactions of neutralization, precipitation, and the like.
So the correct answer is 2.
Example 3
Zn (TV) + 2 H + (solution) → Zn 2+ + H 2 +154 kJ
necessary:
reduce the concentration of zinc ions
increase the concentration of hydrogen ions
decrease the temperature
increase the concentration of zinc ions
Remember that the rate of a chemical reaction depends on
The nature of the reactants
Temperatures
The concentrations of the reactants,
The degree of grinding of solids (from the contact area in the case of heterogeneous reactions),
The presence of a catalyst (inhibitor).
It's obvious that
a change in the concentration of zinc ions (increase or decrease) will not affect the reaction rate in any way, since zinc ions are not reagents, but reaction products;
A decrease in temperature always leads to a decrease in the rate of a chemical reaction.
Therefore, only an increase in the concentration of hydrogen ions (i.e., reagents) will cause an acceleration of a chemical reaction. So the correct answer is 2.
Example 4
To increase the rate of a chemical reaction
Fe (TV) + 2 H + (solution) → Fe 2+ + H 2
necessary:
1) add a few pieces of iron,
2) increase the concentration of iron ions,
3) reduce the temperature,
4) grind the taken iron.
Most of the examinees chose the wrong answer to this question, namely answer 1. Indeed, if not one, but two or three pieces of iron are added to a test tube with acid, the amount of hydrogen released will increase significantly. But will this increase the rate of reaction? Of course not! And that's why. The rate of a heterogeneous reaction is equal to the amount of substance of the reaction product formed per unit area of the contact surface of the reactants (for example, per square centimeter). Regardless of how many pieces of metal are taken for the experiment, per unit surface area of each of them (from 1 cm 2 ) will release the same constant amount of hydrogen. Since the area of three pieces of metal is larger than the area of one piece, more hydrogen will be released, although the reaction rate will not change from this - it will be constant. The correct answer is 4, since the rate of a heterogeneous reaction depends on the contact area of the reactants (the more crushed the substance, the larger the contact area).
Example 5
On the rate of reaction between acetic acid and ethanoldoes not affect :
catalyst
reaction temperature
concentration of starting substances
pressure.
The reasoning is very simple. Acetic acid and ethanol are liquids. Therefore, the rate of reaction between these substances is not affected by a change in pressure. Correct answer 4.
Example 6
Reacts with hydrogen at the highest rate
chlorine
fluorine
sulfur
carbon
To answer the question, let's recall the comparative chemical activity of the non-metals indicated in the task. Carbon and sulfur are low-active non-metals. When heated, their activity increases markedly, but at high temperatures, gaseous hydrogen will interact with solid sulfur (the melting point of sulfur is 444 0 C) and solid carbon. And the rate of reactions between gas and solids, as a rule, is less than with the participation of only gaseous substances. The chemical activity of halogens is much greater than other non-metals (ceteris paribus). The most active of the halogens is fluorine. As you know, even such stable substances as water and fiberglass burn in a fluorine atmosphere. Indeed, hydrogen and chlorine interact either when heated or in bright light, while fluorine and hydrogen explode under any conditions (even at very low temperatures). Correct answer 2.
Example 7
To increase the reaction rate
2CO + O 2 = 2СО 2 +Q
necessary:
increase CO concentration
reduce the concentration of 2
lower the pressure
lower the temperature
It is known that the rate of a chemical reaction depends on the following factors:
The nature of the reacting substances (ceteris paribus, more active substances react faster);
The concentration of reactants (the higher the concentration, the higher the reaction rate);
Temperatures (an increase in temperature leads to an acceleration of the reaction);
The presence of a catalyst (a catalyst speeds up the process, an inhibitor slows it down);
Pressure (for reactions involving gases, an increase in pressure is equivalent to an increase in concentration, so the rate of reactions increases with increasing pressure);
The degree of grinding of solids9 the greater the degree of grinding, the greater the contact surface of the solid reagents, and the higher the reaction rate).
Considering these factors, we analyze the proposed answers:
1) An increase in the concentration of CO (the starting substance) will indeed lead to an increase in the rate of a chemical reaction.
2) Reducing the concentration of O 2 will lead not to an increase, but to a decrease in the reaction rate.
3) A decrease in pressure is essentially the same as a decrease in the concentration of reagents, therefore, the reaction rate will also decrease.
4) A decrease in temperature always leads to a decrease in the rate of a chemical reaction.
So the answer is 1.
Example 8
To increase the rate of interaction of iron with hydrochloric acid, one should
1) add an inhibitor
2) lower the temperature
3) increase pressure
4) increase the concentration of NAL
First of all, we write the equation for the interaction of iron with hydrochloric acid:
Fe (TV) + 2 HCl (rr) = FeCl 2(rr) + H 2(d)
Analyzing the proposed answers, we note that the addition of an inhibitor reduces the rate of reactions, and a decrease in temperature has a similar effect. Pressure does not affect the rate of this reaction (because there are no gaseous substances among the reagents). Therefore, to increase the reaction rate, one should increase the concentration of one of the reactants, namelyHCl. Answer 4.
In custody
The experience of conducting the Unified State Examination shows that, in general, graduates successfully cope with the new form of exams. However, in order to increase the objectivity of the results, it is necessary to carry out special training of students for the final certification, for example, to form the ability to work with various types of test items. To introduce more widely into teaching practice test forms for assessing educational achievements along with traditional methods and forms. It is advisable to organize a repetition of the material covered, especially for the course of the basic school, allocating special time for this in the educational process. The attention of teachers, authors of textbooks and developers of teaching aids should be drawn to the need to improve the educational process, taking into account the results of the USE.
Used Books
Medvedev Yu.N., Kaverina A.A., Koroshchenko A.S. Unified state exam 2007. Chemistry. Educational and training materials for the preparation of students / FIPI - M .: Intellect-Center, 2007
A.A. Kaverina, D.Yu. Dobrotin, A.S. Koroshchenko, Yu.N. Medvedev, M.G. Snap. Passing the state exam. Chemistry - M.: Bustard, 2008
Kaverina A.A., Koroshchenko A.S., Medvedev Yu.N., Yashukova A.V. Unified state exam 2009. Chemistry. Universal materials for the preparation of students / FIPI. – M.: Intellect-Centre, 2009
A.A. Kaverina, Yu.N. Medvedev, D.Yu. Dobrotin. USE 2010. Chemistry: a collection of examination tasks - M .: Eksmo, 2009 (USE. Federal Bank of Exam Materials.
Subject: chemistry
Grade: 9
Topic: The rate of chemical reactions
The purpose of the lesson : creating conditions for awareness and understanding of knowledge on the topic “The rate of chemical reactions. Factors affecting the rate of chemical reactions»»
Lesson objectives:
Educational aspect:
to form the concept of "rate of chemical reactions", as a result of observation, analysis, comparison, generalization, bring students to understand the dependence of the rate of chemical reactions on various factors;
formation of skills of research activity of students.
Development aspect:
contribute to the formation of general educational skills:
educational and intellectual (analyze facts, establish causal relationships, draw conclusions);
educational and informational (work with tests);
educational and organizational (understand the meaning of the task, allocate time to complete test tasks and check them);
educational and communicative (the ability to conduct a dialogue, express one's point of view);
contribute to the development of students' horizons.
Educational aspect:
to promote the education of accuracy, attentiveness, caution when working with laboratory equipment and reagents.
Equipment:
for laboratory work: set of reagents: solution of HCl, Zn in tablets and powder, Fe, Mg; spirit lamps, matches, holders, test tubes;
on the teacher's desk HCl (conc), lithium salt, sugar cube, spirit lamp, matches, crucible tongs;
demonstration computer equipment.
Lesson type: lesson of "discovery" of new knowledge with elements of research
Form of organization: frontal, pair work
The use of pedagogical technologies:
problem learning technology;
technology of collective interaction;
critical thinking technology
case based learning
Teaching methods and techniques:
problem dialogue;
encouraging dialogue;
leading dialogue;
comparative method;
study
During the classes
Teacher activity
Student activities
I. Motivation-target stage.
1. Knowledge update (3 min.)
Today we have an unusual lesson. Tell me, please, do you like juice?
After opening the package, the juice is recommended to be consumed immediately. But it happens that it stays, it costs 2-3 days. As a result, the juice begins to ferment and acquires an unpleasant odor.
What does this indicate (in terms of chemistry)? What signs of chemical reactions are observed in this case?
This means that even in everyday life we are constantly dealing with chemical reactions. And today we will talk about reactions.
2. Goal setting (3 min.)
What has changed in the spoiled juice from a chemical point of view?
But it happens that the juice can stand unchanged, and there are no signs of spoilage.
Does this mean that chemical reactions do not occur?
This means that chemical reactions proceed at different rates. And today we have to get acquainted with the concept of "the rate of a chemical reaction" and identify what factors it depends on.. chemical reactions processes that break old chemical bonds and create new ones.
Write down the topic of the lesson.
II. procedural stage. Joint discovery of new knowledge.
1. The concept of the rate of a chemical reaction. (10 min.) - change of reacting substances per unit of time.
2. Physical education (2 minutes.)
We have a gym again
Bent over, come on, come on!
Stretched out, stretched out
And now they've leaned back.
Head tired too
So let's help her.
Left and right, one and two
Think, think, head.
Although the charge is short,
We rested a bit.
3. Factors affecting the rate of chemical reactions (20 minutes.)
Let's go back to our example.
What can we do to keep the juice as long as possible?
Correctly. So the first factor is:
1) the nature of the reacting substances - the nature of which reagent affects the rate of interaction of the acid with the metal?The essence of the reactions of metals with an acid is that metal atoms donate electrons (i.e., they are oxidized, they are reducing agents) to hydrogen protons (i.e., they are reduced, they are oxidizing agents). But metals have different reducing abilities, this ability is characterized by the standard electrode potential, for zinc it is -0.76 V, for iron - 0.037 V, for hydrogen 0.
2) the concentration of reactants - the higher the concentration, the higher the speed
3) the contact area of the reactants The larger the contact surface area, the greater the rate of the chemical reaction.
4)Temperature
The higher the temperature, the faster the rate of chemical reactions; The lower the temperature, the slower the rate of chemical reactions.
So, we learned what the rate of a chemical reaction is, and what it depends on.
III. Self-test. Correction of the obtained results. (3 min.)
I propose to solve the test, evaluate the knowledge gained. Choose the correct answers.
Self-test: answers: 1. B); 2. B); 3. D); 4. B); 5. D).
IV. Reflection. (2 minutes.)
I hope that during our lesson you learned a lot of new and important things that can be useful in life.
Now I will offer you a little test. Upvote affirmations.
I have learned a lot of new things.
I will need this in my life.
There was a lot to think about in class.
I received answers to all the questions that I had.
I worked hard in class.
Count the number of pluses. Their number tells you how you worked in the lesson.
V. Homework. (Slide 15) (2 minutes.)
1. Solve the problem: Determine the rate of the chemical reaction H2 +Br2 = 2HBr if the initial concentration of hydrogen was 1 mol/l, and after 30 seconds it became 0.8 mol/l. (The task is printed on leaflets)
2. Give examples of the influence of various factors on the rate of chemical reactions that you carry out at home, in everyday life.
Answer(usually yes )
Answer:
- A chemical reaction is taking place
- Emission of gas, odor
The rate of a chemical reaction is the change in the concentration of a substance per unit time, where the concentration C is expressed in mol / l. The USE does not require the use of this formula, it is only necessary to be able to compare the rates of various reactions and determine how they are affected by changes in external conditions.
The reaction rate depends on several factors:
- - The nature of the interacting substances
- - Temperatures
- - Concentrations of reactants
- - The presence of a catalyst or inhibitor in the reaction mixture
- - Areas of contact surface of reagents (for heterogeneous processes).
Let's consider each item separately:
1) The nature of the reagents
Obviously, different reactions under the same conditions proceed at different rates. The neutralization of alkali with acid proceeds almost instantly, the dissolution of zinc in hydrochloric acid is fast, the rusting of iron under the action of water and oxygen is much longer. The general rule is obvious: the more active the reacting substances, the faster they interact with each other.
The fastest reactions are homogeneous flowing in one phase (gases or miscible liquids). In them, the interaction occurs in the entire volume of the mixture of reagents.
heterogeneous reactions- the interaction of substances that do not mix with each other - proceed at the interface. These processes slower, and the speed is determined by the area of the contact surface.
2) Temperature
As the temperature increases, the molecules begin to move faster, therefore, they collide with each other more often. In this way, The rate of a reaction always increases with increasing temperature and decreases with decreasing temperature. We actively use this in everyday life: for example, to slow down the processes of decay (complex chemical reactions), we store food in the refrigerator, and to speed up the Maillard reaction (the interaction of proteins with sugars), we cook food at high temperatures.
The dependence of the reaction rate on temperature is determined by the van't Hoff equation: where v 2 and v 1 are the reaction rates at temperatures T 2 and T 1, respectively, γ is the temperature coefficient, which takes values from 2 to 4. For example, if γ \u003d 3, at raising the temperature by 20 degrees will increase the rate of the reaction.
3) Concentration of reagents
It's obvious that the higher the concentration of reactants, the more often their molecules will collide with each other, therefore, the faster the interaction. The dependence of the velocity on the concentration of reagents is expressed by the law of mass action.
For example, for the reaction aA + bB = cC + dD the speed is . In fact, often the dependence of the rate on the concentrations of the reactants is more complex, since many processes proceed through the formation of intermediate products, but we leave these cases to the university course.
Please do not confuse: The reaction rate depends on the concentrations of the reactants and does not depend on the concentrations of the products!
Here we consider the effect of pressure on the reaction rate. Liquids and solids are practically incompressible and pressure does not affect their concentrations. For gases, an increase in pressure causes an increase in concentration, hence speeding up the reaction.
4) Catalysts and inhibitors
A catalyst is a substance that increases the rate of a reaction, but is not consumed in it, an inhibitor is a reverse catalyst, it slows down the reaction.
5) Interface area
For example, consider the reaction of zinc with hydrochloric acid:
Zn + 2HCl \u003d ZnCl 2 + H 2.
A piece of zinc will gradually dissolve in acid: first, the top layer of molecules will react, then the next one, and so on. "Internal" atoms will be able to react only after they go into solution "external".
If this piece is crushed (that is, its surface area is increased), the reaction will go much faster, because. the number of "acid-accessible" particles will increase.
In this way, the more finely divided the solid, the faster the reaction rate. The amount of solid matter affects the rate much weaker than the degree of its grinding and the concentration of liquid or gaseous reagents.
Let's practice. Below are questions from the exam from different years:
| A) The rate of the chemical reaction 2C (tv) + CO2 (g) \u003d 2CO (g) does not depend from | 1) temperature 2) CO concentration 3) the degree of grinding of coal 4) pressure |
||||||||
| B) The reaction between honey and hydrogen proceeds at the highest rate at room temperature | 1) gray | ||||||||
| C) To increase the rate of the chemical reaction Mg (tv) + 2HCl (solution) \u003d MgCl2 (solution) + H2 (g) it is necessary | 1) increase pressure 2) reduce the temperature 3) increase the concentration of HCl 4) increase the amount of magnesium. |
||||||||
| D) Increasing the pressure will increase the reaction rate | 1) 2P (tv) + 3S (tv) = P2S3 (tv) 2) Br2 (l) + 2Na (tv) = 2NaBr 3) H2O (l) + 2Na (tv) = 2NaOH + H2 (g) 4) N2 (g) + 3H2 (g) = 2NH3 (g) |
||||||||
Answers:
|
|||||||||